当前位置:
X-MOL 学术
›
Arch. Biochem. Biophys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of silicon nanocone on cell membrane self-sealing capabilities for targeted drug delivery—Computer simulation study
Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2023-10-31 , DOI: 10.1016/j.abb.2023.109802 Przemysław Raczyński 1 , Krzysztof Górny 1 , Piotr Bełdowski 2 , Beata Marciniak 3 , Thorsten Pöschel 4 , Zbigniew Dendzik 1
Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2023-10-31 , DOI: 10.1016/j.abb.2023.109802 Przemysław Raczyński 1 , Krzysztof Górny 1 , Piotr Bełdowski 2 , Beata Marciniak 3 , Thorsten Pöschel 4 , Zbigniew Dendzik 1
Affiliation
|
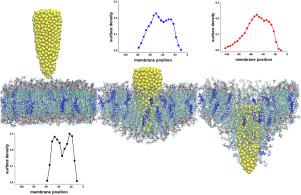
Efficient and non-invasive techniques of cargo delivery to biological cells are the focus of biomedical research because of their great potential importance for targeted drug therapy. Therefore, much effort is being made to study the characteristics of using nano-based biocompatible materials as systems that can facilitate this task while ensuring appropriate self-sealing of the cell membrane. Here, we study the effects of indentation and withdrawal of nanocone on phospholipid membrane by applying steered molecular dynamics (SMD) technique. Our results show that the withdrawal process directly depends on the initial position of the nanocone. The average force and work are considerably more significant in case of the withdrawal starting from a larger depth. This result is attributed to stronger hydrophobic interactions between the nanocone and lipid tails of the membrane molecules. Furthermore, when the indenter was started from the lower initial depth, the number of lipids removed from the membrane was several times smaller than the deeper indentation. The choice of the least invasive method for nanostructure-assisted drug delivery is crucial for possible applications in medicine. Therefore, the results presented in this work might be helpful in efficient and safe drug delivery with nanomaterials.
中文翻译:

硅纳米锥对靶向药物递送细胞膜自密封能力的影响——计算机模拟研究
将货物运送到生物细胞的高效和非侵入性技术是生物医学研究的重点,因为它们在靶向药物治疗中具有巨大的潜在重要性。因此,人们正在努力研究使用纳米基生物相容性材料作为系统的特点,这些材料可以促进这项任务,同时确保细胞膜的适当自密封。在这里,我们通过应用受控分子动力学 (SMD) 技术研究纳米锥的压痕和退出对磷脂膜的影响。我们的结果表明,撤离过程直接取决于纳米锥的初始位置。如果从更大的深度开始撤退,则平均力和功要重要得多。这一结果归因于膜分子的纳米锥和脂质尾部之间更强的疏水相互作用。此外,当压头从较低的初始深度开始时,从膜中去除的脂质数量比较深的压痕少几倍。选择微创的纳米结构辅助药物递送方法对于可能的医学应用至关重要。因此,这项工作中提出的结果可能有助于使用纳米材料进行高效和安全的药物递送。
更新日期:2023-10-31
中文翻译:

硅纳米锥对靶向药物递送细胞膜自密封能力的影响——计算机模拟研究
将货物运送到生物细胞的高效和非侵入性技术是生物医学研究的重点,因为它们在靶向药物治疗中具有巨大的潜在重要性。因此,人们正在努力研究使用纳米基生物相容性材料作为系统的特点,这些材料可以促进这项任务,同时确保细胞膜的适当自密封。在这里,我们通过应用受控分子动力学 (SMD) 技术研究纳米锥的压痕和退出对磷脂膜的影响。我们的结果表明,撤离过程直接取决于纳米锥的初始位置。如果从更大的深度开始撤退,则平均力和功要重要得多。这一结果归因于膜分子的纳米锥和脂质尾部之间更强的疏水相互作用。此外,当压头从较低的初始深度开始时,从膜中去除的脂质数量比较深的压痕少几倍。选择微创的纳米结构辅助药物递送方法对于可能的医学应用至关重要。因此,这项工作中提出的结果可能有助于使用纳米材料进行高效和安全的药物递送。

































 京公网安备 11010802027423号
京公网安备 11010802027423号