当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insights into the inhibitory mechanism of angiotensin-I-converting enzyme by the lactotripeptides IPP and VPP
FEBS Letters ( IF 3.0 ) Pub Date : 2023-10-30 , DOI: 10.1002/1873-3468.14768 Kyle S Gregory 1 , Gyles E Cozier 1 , Sylva L U Schwager 2 , Edward D Sturrock 2 , K Ravi Acharya 1
FEBS Letters ( IF 3.0 ) Pub Date : 2023-10-30 , DOI: 10.1002/1873-3468.14768 Kyle S Gregory 1 , Gyles E Cozier 1 , Sylva L U Schwager 2 , Edward D Sturrock 2 , K Ravi Acharya 1
Affiliation
|
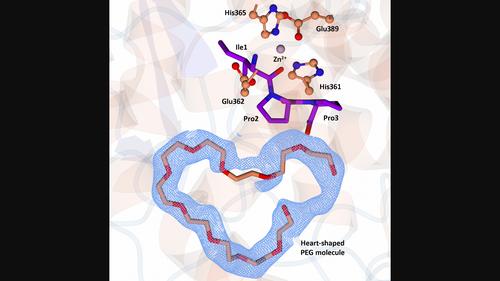
Human somatic angiotensin-1-converting enzyme (sACE) is composed of a catalytic N-(nACE) and C-domain (cACE) of similar size with different substrate specificities. It is involved in the regulation of blood pressure by converting angiotensin I to the vasoconstrictor angiotensin II and has been a major focus in the development of therapeutics for hypertension. Bioactive peptides from various sources, including milk, have been identified as natural ACE inhibitors. We report the structural basis for the role of two lacototripeptides, Val-Pro-Pro and Ile-Pro-Pro, in domain-specific inhibition of ACE using X-ray crystallography and kinetic analysis. The lactotripeptides have preference for nACE due to altered polar interactions distal to the catalytic zinc ion. Elucidating the mechanism of binding and domain selectivity of these peptides also provides important insights into the functional roles of ACE.
中文翻译:

乳三肽 IPP 和 VPP 抑制血管紧张素 I 转换酶机制的结构见解
人体血管紧张素 1 转换酶 (sACE) 由大小相似但底物特异性不同的催化 N 结构域 (nACE) 和 C 结构域 (cACE) 组成。它通过将血管紧张素 I 转化为血管收缩剂血管紧张素 II 来参与血压调节,并且一直是高血压治疗方法开发的主要焦点。来自各种来源(包括牛奶)的生物活性肽已被确定为天然 ACE 抑制剂。我们使用 X 射线晶体学和动力学分析报告了两种乳三肽 Val-Pro-Pro 和 Ile-Pro-Pro 在 ACE 域特异性抑制中的作用的结构基础。由于催化锌离子远端极性相互作用的改变,乳三肽优先选择 nACE。阐明这些肽的结合机制和结构域选择性也为了解 ACE 的功能作用提供了重要的见解。
更新日期:2023-10-30
中文翻译:

乳三肽 IPP 和 VPP 抑制血管紧张素 I 转换酶机制的结构见解
人体血管紧张素 1 转换酶 (sACE) 由大小相似但底物特异性不同的催化 N 结构域 (nACE) 和 C 结构域 (cACE) 组成。它通过将血管紧张素 I 转化为血管收缩剂血管紧张素 II 来参与血压调节,并且一直是高血压治疗方法开发的主要焦点。来自各种来源(包括牛奶)的生物活性肽已被确定为天然 ACE 抑制剂。我们使用 X 射线晶体学和动力学分析报告了两种乳三肽 Val-Pro-Pro 和 Ile-Pro-Pro 在 ACE 域特异性抑制中的作用的结构基础。由于催化锌离子远端极性相互作用的改变,乳三肽优先选择 nACE。阐明这些肽的结合机制和结构域选择性也为了解 ACE 的功能作用提供了重要的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号