当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Determination of the Mechanism of the Palladium-Catalyzed Domino Reaction of ortho-Iodostyrene, Oxanorbornadiene, and Phenylboronic Acid
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-10-31 , DOI: 10.1021/acs.joc.3c01522 Chunyu Han 1 , Fengyue Zhao 1 , Qianqian Lu 1 , Fang Liu 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-10-31 , DOI: 10.1021/acs.joc.3c01522 Chunyu Han 1 , Fengyue Zhao 1 , Qianqian Lu 1 , Fang Liu 1, 2
Affiliation
|
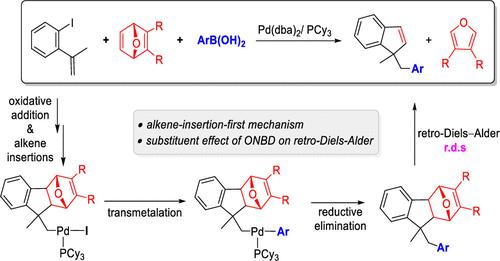
The palladium-catalyzed three-component domino reaction of ortho-iodostyrene, 2,3-dicarbomethoxy-7-oxanorbornadiene (ONBD), and phenylboronic acid discovered by the Lautens group provides a convenient method to synthesize indenes derivatives. Herein, density functional theory (DFT) calculations were employed to explore the detailed mechanism of this domino reaction. The computational results suggest that the alkene-insertion-first and the transmetalation-first mechanisms are competitive, and the former mechanism is slightly more favorable because of the difficult intramolecular alkene insertion of the alkyl-PdII-aryl than alkyl-PdII-I complex. Further analysis on substituents of ONBD unveils the impacts of noncovalent interactions and electronic effect on the rate-determining retro-Diels–Alder process. The understanding of this domino reaction has important implications for developing a novel palladium-catalyzed domino reaction with a retro-Diels–Alder strategy.
中文翻译:

钯催化邻碘苯乙烯、氧降冰片二烯和苯基硼酸多米诺反应机理的计算确定
Lautens小组发现的钯催化的邻碘苯乙烯、2,3-二甲氧基-7-氧杂降冰片二烯(ONBD)和苯基硼酸的三组分多米诺反应为合成茚衍生物提供了一种简便的方法。在此,采用密度泛函理论(DFT)计算来探索这种多米诺骨牌反应的详细机制。计算结果表明,烯烃先插入机制和先金属转移机制是竞争性的,并且前一种机制稍微更有利,因为烷基-Pd II -芳基的分子内烯烃插入比烷基-Pd II -I困难。复杂的。对 ONBD 取代基的进一步分析揭示了非共价相互作用和电子效应对速率决定的逆狄尔斯-阿尔德过程的影响。对这种多米诺骨牌反应的理解对于开发一种采用逆狄尔斯-阿尔德策略的新型钯催化多米诺骨牌反应具有重要意义。
更新日期:2023-10-31
中文翻译:

钯催化邻碘苯乙烯、氧降冰片二烯和苯基硼酸多米诺反应机理的计算确定
Lautens小组发现的钯催化的邻碘苯乙烯、2,3-二甲氧基-7-氧杂降冰片二烯(ONBD)和苯基硼酸的三组分多米诺反应为合成茚衍生物提供了一种简便的方法。在此,采用密度泛函理论(DFT)计算来探索这种多米诺骨牌反应的详细机制。计算结果表明,烯烃先插入机制和先金属转移机制是竞争性的,并且前一种机制稍微更有利,因为烷基-Pd II -芳基的分子内烯烃插入比烷基-Pd II -I困难。复杂的。对 ONBD 取代基的进一步分析揭示了非共价相互作用和电子效应对速率决定的逆狄尔斯-阿尔德过程的影响。对这种多米诺骨牌反应的理解对于开发一种采用逆狄尔斯-阿尔德策略的新型钯催化多米诺骨牌反应具有重要意义。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号