当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron Donation from Boron Suboxides via Strong p–d Orbital Hybridization Boosts Molecular O2 Activation on Ru/TiO2 for Low-Temperature Dibromomethane Oxidation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-10-31 , DOI: 10.1021/acs.est.3c04725 Guanqun Gao 1 , Wei Liu 2, 3 , Zhisong Liu 1, 4 , Zihao Li 1 , Haomiao Xu 1 , Wenjun Huang 1 , Naiqiang Yan 1, 5 , Zan Qu 1, 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-10-31 , DOI: 10.1021/acs.est.3c04725 Guanqun Gao 1 , Wei Liu 2, 3 , Zhisong Liu 1, 4 , Zihao Li 1 , Haomiao Xu 1 , Wenjun Huang 1 , Naiqiang Yan 1, 5 , Zan Qu 1, 5
Affiliation
|
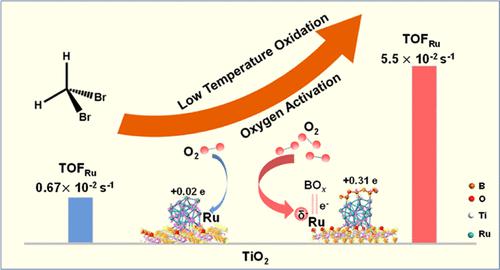
Low-temperature catalytic oxidation is of significance to the degradation of halogenated volatile organic compounds (HVOCs) to avoid hazardous byproducts with low energy consumption. Efficient molecular oxygen (O2) activation is pivotal to it but usually limited by the insufficient electron cloud density at the metal center. Herein, Ru–B catalysts with enhanced electron density around Ru were designed to achieve efficient O2 activation, realizing dibromomethane (DBM) degradation T90 at 182 °C on RuB1/TiO2 (about 30 °C lower than pristine Ru/TiO2) with a TOFRu value of 0.055 s–1 (over 8 times that of Ru/TiO2). Compared to the limited electron transfer (0.02 e) on pristine Ru/TiO2, the Ru center gained sufficient negative charges (0.31 e) from BOx via strong p–d orbital hybridization. The Ru–B site then acted as the electron donor complexing with the 2π* antibonding orbital of O2 to realize the O2 dissociative activation. The reactive oxygen species formed thereby could initiate a fast conversion and oxidation of formate intermediates, thus eventually boosting the low-temperature catalytic activity. Furthermore, we found that the Ru–B sites for O2 activation have adaptation for pollutant removal and multiple metal availability. Our study shed light on robust O2 activation catalyst design based on electron density adjustment by boron.
中文翻译:

低氧化硼通过强 p-d 轨道杂化提供电子促进 Ru/TiO2 上的分子 O2 活化,用于低温二溴甲烷氧化
低温催化氧化对于卤代挥发性有机化合物(HVOCs)的降解以避免产生危险副产物具有重要意义,并且能耗低。有效的分子氧(O 2)活化对其至关重要,但通常受到金属中心电子云密度不足的限制。本文中,Ru-B催化剂在Ru周围具有增强的电子密度,旨在实现有效的O 2活化,在RuB 1 /TiO 2上于182°C下实现二溴甲烷(DBM)降解T 90(比原始Ru/TiO低约30°C)2 ),TOF Ru值为 0.055 s –1(超过 Ru/TiO 2的 8 倍)。与原始Ru/TiO 2上有限的电子转移(0.02 e)相比,Ru中心通过强p-d轨道杂化从BO x获得了足够的负电荷(0.31 e)。然后Ru-B位点作为电子供体与O 2的2π*反键轨道络合,实现O 2解离活化。由此形成的活性氧可以引发甲酸盐中间体的快速转化和氧化,从而最终提高低温催化活性。此外,我们发现用于 O 2活化的 Ru-B 位点具有污染物去除和多种金属可用性的适应性。我们的研究揭示了基于硼调整电子密度的稳健 O 2活化催化剂设计。
更新日期:2023-10-31
中文翻译:

低氧化硼通过强 p-d 轨道杂化提供电子促进 Ru/TiO2 上的分子 O2 活化,用于低温二溴甲烷氧化
低温催化氧化对于卤代挥发性有机化合物(HVOCs)的降解以避免产生危险副产物具有重要意义,并且能耗低。有效的分子氧(O 2)活化对其至关重要,但通常受到金属中心电子云密度不足的限制。本文中,Ru-B催化剂在Ru周围具有增强的电子密度,旨在实现有效的O 2活化,在RuB 1 /TiO 2上于182°C下实现二溴甲烷(DBM)降解T 90(比原始Ru/TiO低约30°C)2 ),TOF Ru值为 0.055 s –1(超过 Ru/TiO 2的 8 倍)。与原始Ru/TiO 2上有限的电子转移(0.02 e)相比,Ru中心通过强p-d轨道杂化从BO x获得了足够的负电荷(0.31 e)。然后Ru-B位点作为电子供体与O 2的2π*反键轨道络合,实现O 2解离活化。由此形成的活性氧可以引发甲酸盐中间体的快速转化和氧化,从而最终提高低温催化活性。此外,我们发现用于 O 2活化的 Ru-B 位点具有污染物去除和多种金属可用性的适应性。我们的研究揭示了基于硼调整电子密度的稳健 O 2活化催化剂设计。













































 京公网安备 11010802027423号
京公网安备 11010802027423号