Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Dynamics of Confined Water Inside Diphenylalanine Peptide Nanotubes
ACS Omega ( IF 3.7 ) Pub Date : 2023-10-30 , DOI: 10.1021/acsomega.3c06071 Jinfeng Chen 1, 2, 3 , Zongyang Qiu 2, 3 , Jing Huang 2, 3
ACS Omega ( IF 3.7 ) Pub Date : 2023-10-30 , DOI: 10.1021/acsomega.3c06071 Jinfeng Chen 1, 2, 3 , Zongyang Qiu 2, 3 , Jing Huang 2, 3
Affiliation
|
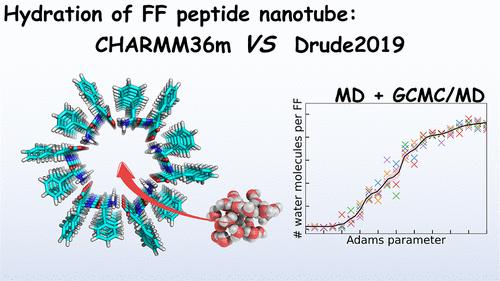
Diphenylalanine (FF) peptides exhibit a unique ability to self-assemble into nanotubes with confined water molecules playing pivotal roles in their structure and function. This study investigates the structure and dynamics of diphenylalanine peptide nanotubes (FFPNTs) using all-atom molecular dynamics (MD) and grand canonical Monte Carlo combined with MD (GCMC/MD) simulations with both the CHARMM additive and Drude polarizable force fields. The occupancy and dynamics of confined water molecules were also examined. It was found that less than 2 confined water molecules per FF help stabilize the FFPNTs on the x–y plane. Analyses of the kinetics of confined water molecules revealed distinctive transport behaviors for bound and free water, and their respective diffusion coefficients were compared. Our results validate the importance of polarizable force field models in studying peptide nanotubes and provide insights into our understanding of nanoconfined water.
中文翻译:

二苯丙氨酸肽纳米管内受限水的结构与动力学
二苯丙氨酸 (FF) 肽表现出独特的自组装成纳米管的能力,其中受限的水分子在其结构和功能中发挥着关键作用。本研究利用全原子分子动力学 (MD) 和大正则蒙特卡罗结合 MD (GCMC/MD) 模拟以及 CHARMM 添加剂和 Drude 极化力场,研究二苯丙氨酸肽纳米管 (FFPNT) 的结构和动力学。还检查了受限水分子的占据和动态。研究发现,每个 FF 少于 2 个受限水分子有助于稳定x – y平面上的 FFPNT。对受限水分子的动力学分析揭示了结合水和自由水的独特传输行为,并比较了它们各自的扩散系数。我们的结果验证了极化力场模型在研究肽纳米管中的重要性,并为我们对纳米约束水的理解提供了见解。
更新日期:2023-10-30
中文翻译:

二苯丙氨酸肽纳米管内受限水的结构与动力学
二苯丙氨酸 (FF) 肽表现出独特的自组装成纳米管的能力,其中受限的水分子在其结构和功能中发挥着关键作用。本研究利用全原子分子动力学 (MD) 和大正则蒙特卡罗结合 MD (GCMC/MD) 模拟以及 CHARMM 添加剂和 Drude 极化力场,研究二苯丙氨酸肽纳米管 (FFPNT) 的结构和动力学。还检查了受限水分子的占据和动态。研究发现,每个 FF 少于 2 个受限水分子有助于稳定x – y平面上的 FFPNT。对受限水分子的动力学分析揭示了结合水和自由水的独特传输行为,并比较了它们各自的扩散系数。我们的结果验证了极化力场模型在研究肽纳米管中的重要性,并为我们对纳米约束水的理解提供了见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号