Synlett ( IF 1.7 ) Pub Date : 2023-10-30 , DOI: 10.1055/a-2181-0453 Zhiwei Zeng 1 , Zichang Zhao 1 , Zhaoyang Yin 1 , Mingjie Tang 1 , Yuji Liu 1 , Wei Huang 1 , Yongxing Tang 1
|
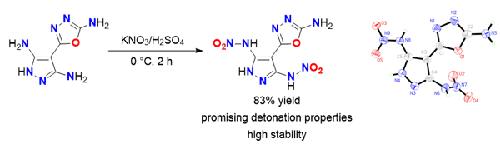
The introduction of nitroamino groups onto a nitrogen-rich heterocyclic skeleton is an efficient method for constructing high-performance energetic compounds. In this work, the nitroamino-functionalized compound 4-(5-amino-1,3,4-oxadiazol-2-yl)-N,N′-dinitro-1H-pyrazole-3,5-diamine was synthesized in yields of up to 83%. In addition, its energetic salts were also prepared. All these compounds were characterized by NMR and IR spectroscopy and, in two cases, by single-crystal X-ray diffraction. In addition, the difference in the reactivities of the three amino groups on the pyrazolyloxadiazole system was analyzed by an average local ionization energy analysis. The hydrazinium salt of the diamine exhibits promising detonation properties and good molecular stability, suggesting it has good application potential. Compared with previous works, this strategy gives an improved isolated yield and provides a promising method for the construction of nitroamino-functionalized 1,3,4-oxadiazole derivatives.
中文翻译:

在吡唑基-1,3,4-恶二唑框架上组装硝基氨基和氨基用于构建高性能和不敏感的含能材料
在富氮杂环骨架上引入硝基氨基是构建高性能含能化合物的有效方法。本工作中,合成了硝基氨基官能化化合物4-(5-氨基-1,3,4-恶二唑-2-基)-N , N'-二硝基-1H-吡唑-3,5-二胺,收率高达83%。此外,还制备了其含能盐。所有这些化合物都通过核磁共振和红外光谱进行了表征,在两种情况下还通过单晶 X 射线衍射进行了表征。此外,通过平均局部电离能分析分析了吡唑恶二唑体系上三个氨基的反应活性差异。二胺肼盐表现出良好的爆轰性能和良好的分子稳定性,表明其具有良好的应用潜力。与之前的工作相比,该策略提高了分离产率,并为构建硝基氨基功能化的1,3,4-恶二唑衍生物提供了一种有前途的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号