Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-10-30 , DOI: 10.1016/j.bioorg.2023.106950 Qiuping Xiang 1 , Tianbang Wu 2 , Cheng Zhang 3 , Chao Wang 3 , Hongrui Xu 4 , Qingqing Hu 3 , Jiankang Hu 5 , Guolong Luo 3 , Xiaoxi Zhuang 3 , Xishan Wu 3 , Yan Zhang 3 , Yong Xu 6
|
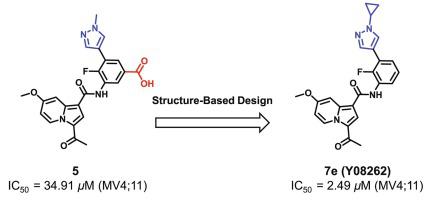
The bromodomain of CREB (cyclic-AMP response element binding protein) binding protein (CBP) is an epigenetic “reader” and plays a key role in transcriptional regulation. CBP bromodomain is considered to be a promising therapeutic target for acute myeloid leukemia (AML). Herein, we report the discovery of a series of 1-(indolizin-3-yl)ethan-1-one derivatives as potent, and selective CBP bromodomain inhibitors focused on improving cellular potency. One of the most promising compounds, 7e (Y08262), inhibits the CBP bromodomain at the nanomolar level (IC50 = 73.1 nM) with remarkable selectivity. In addition, the new inhibitor also displays potent inhibitory activities in AML cell lines. Collectively, this study provides a new lead compound for further validation of CBP bromodomain as a molecular target for AML drug development.
中文翻译:

发现一种有效的选择性 CBP 溴结构域抑制剂 (Y08262) 治疗急性髓性白血病
CREB (cyclic-AMP response element binding protein) 结合蛋白 (CBP) 的溴结构域是表观遗传学“读取器”,在转录调控中起关键作用。CBP 溴结构域被认为是急性髓性白血病 (AML) 的一个有前途的治疗靶点。在此,我们报道了一系列 1-(吲哚嗪-3-基)乙-1-酮衍生物的发现,这些衍生物是专注于提高细胞效力的有效和选择性 CBP 溴结构域抑制剂。最有前途的化合物之一 7e (Y08262) 在纳摩尔水平 (IC50 = 73.1 nM) 抑制 CBP 溴结构域,具有显著的选择性。此外,新抑制剂在 AML 细胞系中也显示出有效的抑制活性。总的来说,本研究为进一步验证 CBP 溴结构域作为 AML 药物开发的分子靶点提供了一种新的先导化合物。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号