Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-10-30 , DOI: 10.1016/j.cej.2023.147010 Mengjie Zhao , Khalid A.M. Salih , Yuezhou Wei , Eric Guibal , Shunyan Ning , Adel E.-S. Goda , Mohammed F. Hamza
|
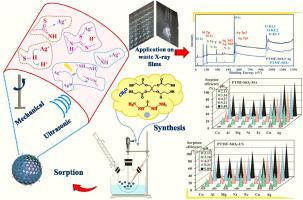
The reaction of thiocarbazide with pentaerythritol tetrakis(3-mercaptopropionate) in the presence of mesoporous silica beads allows producing a composite sorbent (PTMF-SiO2) with high affinity for silver. The high density of both N- and S-based reactive groups (about 5 mmol g−1) explains sorption capacities as high as 3 mmol Ag g−1 at pH 6. The sorption mechanisms are correlated to the pH effect, and to FTIR and XPS spectroscopic analyses. The sorption is endothermic and spontaneous, and the sorption isotherms are successfully modeled using the Temkin equation. Uptake kinetics are fitted by the pseudo-first order rate equation: the equilibrium is reached in 20–30 min. Ultrasonic treatment allows slightly increasing both kinetics (apparent rate coefficient k1 is almost doubled) and sorption capacities (by less than 7 %). Silver (being a soft acid) preferentially reacts with soft bases; this may explain the selective removal of Ag(I) by PTMF-SiO2 against base metals and alkali or alkaline earth metals (in multicomponent equimolar solutions). The sorbent shows remarkable recycling stability (for at least 5 cycles): complete desorption is achieved in ≈20 min with 0.3 M HNO3 solution and the loss in sorption does not exceed 2.5 % at the fifth recycling. Finally, the sorbent is successfully applied to silver recovery from acidic leachates of spent X-ray films (from different hospitals from Hengyang, China), as a case study. Silver is selectively recovered with high efficiency (>96 %) (together with Cu(II), to a lesser extent).


















































 京公网安备 11010802027423号
京公网安备 11010802027423号