当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot access to 11-methyl-6H-indolo[2,3-b]quinolines via iodine-mediated annulation of indoles with 2-vinylanilines and evaluation of their biological activities
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-10-30 , DOI: 10.1039/d3qo01319g Jinglan Yan 1 , Zeguo Fang 1 , Jianglong Su 1 , Qun He 1 , Nawaf Al-Maharik 2 , Qian Zhang 1 , Yanhong Wei 1 , Dong Li 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-10-30 , DOI: 10.1039/d3qo01319g Jinglan Yan 1 , Zeguo Fang 1 , Jianglong Su 1 , Qun He 1 , Nawaf Al-Maharik 2 , Qian Zhang 1 , Yanhong Wei 1 , Dong Li 1
Affiliation
|
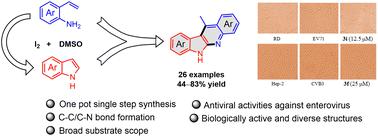
A simple, straightforward and efficient method for the synthesis of a series of 11-methyl-6H-indolo[2,3-b]quinolines bearing different substituents on the indole and quinoline rings is described. The method involved an annulation reaction of indoles with 2-vinylanilines in the presence of iodine through a cascade intermolecular nucleophilic substitution and intramolecular cyclization in one pot. This process does not require any pre-functionalization procedures for the new C–C and C–N bond formation and provides the desired products in moderate to good yields. Additionally, these tetracyclic compounds were evaluated for their cytotoxicity and antiviral activity against viruses EV71 and CVB3. The preliminary results showed that some of them exhibited excellent antiviral effects on EV71 and CVB3, along with the ability to effectively inhibit virus-induced cytopathic effects and reduce viral progeny yields.
中文翻译:

通过碘介导的吲哚与2-乙烯基苯胺成环一锅法获得11-甲基-6H-吲哚并[2,3-b]喹啉并评估其生物活性
描述了一种简单、直接且有效的合成一系列在吲哚和喹啉环上带有不同取代基的11-甲基-6H-吲哚并[2,3- b ]喹啉的方法。该方法涉及在碘存在下通过级联分子间亲核取代和分子内环化一锅进行吲哚与2-乙烯基苯胺的环化反应。该过程不需要任何预功能化程序来形成新的 C-C 和 C-N 键,并以中等至良好的产率提供所需的产品。此外,还评估了这些四环化合物的细胞毒性和针对病毒 EV71 和 CVB3 的抗病毒活性。初步结果表明,其中一些对EV71和CVB3表现出优异的抗病毒作用,同时能够有效抑制病毒诱导的细胞病变效应并降低病毒后代产量。
更新日期:2023-10-30
中文翻译:

通过碘介导的吲哚与2-乙烯基苯胺成环一锅法获得11-甲基-6H-吲哚并[2,3-b]喹啉并评估其生物活性
描述了一种简单、直接且有效的合成一系列在吲哚和喹啉环上带有不同取代基的11-甲基-6H-吲哚并[2,3- b ]喹啉的方法。该方法涉及在碘存在下通过级联分子间亲核取代和分子内环化一锅进行吲哚与2-乙烯基苯胺的环化反应。该过程不需要任何预功能化程序来形成新的 C-C 和 C-N 键,并以中等至良好的产率提供所需的产品。此外,还评估了这些四环化合物的细胞毒性和针对病毒 EV71 和 CVB3 的抗病毒活性。初步结果表明,其中一些对EV71和CVB3表现出优异的抗病毒作用,同时能够有效抑制病毒诱导的细胞病变效应并降低病毒后代产量。













































 京公网安备 11010802027423号
京公网安备 11010802027423号