当前位置:
X-MOL 学术
›
Chin. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioinspired synthesis of cochlearol B and ganocin A
Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2023-10-30 , DOI: 10.1016/j.cclet.2023.109247
Zhenhao Wang , Yuliang Tang , Ruyu Li , Shuai Tian , Yu Tang , Dehai Li
Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2023-10-30 , DOI: 10.1016/j.cclet.2023.109247
Zhenhao Wang , Yuliang Tang , Ruyu Li , Shuai Tian , Yu Tang , Dehai Li
|
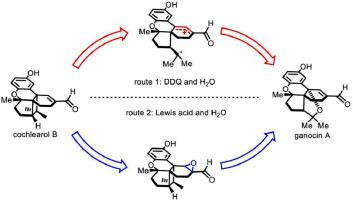
Described here is a divergent, biosynthetically inspired synthesis of cochlearol B and ganocin A. Key steps of the synthesis include the chromene unit construction through a biomimetic acid-catalyzed [4 + 2] ring cyclization. A photochemical [2 + 2] cycloaddition was featured to construct the cyclobutane core of cochlearol B. Different skeletal rearrangements of cochlearol B afforded ganocin A, that one of them was Lewis acid mediated epoxide rearrangement and another was DDQ induced cyclobutane formed tetrahydrofuran ring. The described syntheses not only achieved these natural products in an efficient manner, but also provided insight into the biosynthetic relationship between the two different skeletons.
中文翻译:

仿生合成耳蜗醇 B 和甘诺辛 A
这里描述的是一种不同的、受生物合成启发的耳蜗醇 B 和甘诺辛 A 的合成。合成的关键步骤包括通过仿生酸催化的 [4 + 2] 环环化构建色烯单元。通过光化学[2+2]环加成反应构建了cochlearol B的环丁烷核心。cochlearol B的不同骨架重排得到了ganocin A,其中一种是Lewis酸介导的环氧化物重排,另一种是DDQ诱导环丁烷形成四氢呋喃环。所描述的合成不仅以有效的方式获得了这些天然产物,而且还提供了对两种不同骨架之间的生物合成关系的深入了解。
更新日期:2023-10-30
中文翻译:

仿生合成耳蜗醇 B 和甘诺辛 A
这里描述的是一种不同的、受生物合成启发的耳蜗醇 B 和甘诺辛 A 的合成。合成的关键步骤包括通过仿生酸催化的 [4 + 2] 环环化构建色烯单元。通过光化学[2+2]环加成反应构建了cochlearol B的环丁烷核心。cochlearol B的不同骨架重排得到了ganocin A,其中一种是Lewis酸介导的环氧化物重排,另一种是DDQ诱导环丁烷形成四氢呋喃环。所描述的合成不仅以有效的方式获得了这些天然产物,而且还提供了对两种不同骨架之间的生物合成关系的深入了解。































 京公网安备 11010802027423号
京公网安备 11010802027423号