当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Reaction Chemistry of 1,1,3,3-Tetramethyldisilazane as a Precursor Gas in a Catalytic Chemical Vapor Deposition Process
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-10-26 , DOI: 10.1021/acs.jpca.3c04761 James Michael Stevenson 1 , Eric Ampong 1 , Yujun Shi 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-10-26 , DOI: 10.1021/acs.jpca.3c04761 James Michael Stevenson 1 , Eric Ampong 1 , Yujun Shi 1
Affiliation
|
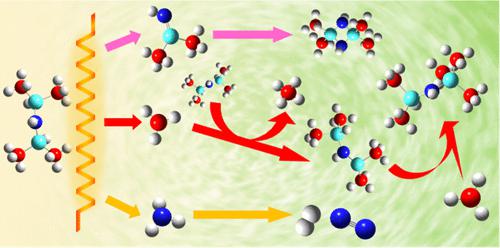
The reaction chemistry of 1,1,3,3-tetramethyldisilazane (TMDSZ) in catalytic chemical vapor deposition (Cat-CVD), including its primary decomposition on a heated W filament and secondary gas-phase reactions in a Cat-CVD reactor, was studied using 10.5 eV vacuum ultraviolet single-photon ionization and/or laser-induced electron ionization in tandem with time-of-flight mass spectrometry. It has been demonstrated that TMDSZ initially breaks down to form various species, including methyl radical (•CH3), ammonia (NH3), and 1,1-dimethylsilanimine (DMSA). The activation energies (Ea) for the formation of •CH3 and NH3 were determined to be 61.2 ± 1.0 and 42.1 ± 0.9 kJ mol–1, respectively, in the temperature range of 1400–2000 and 900–2400 °C. It was found that the formation of DMSA may have two different contributing routes, i.e., a concerted one (Ea = 33.6 ± 2.3 kJ mol–1) at lower temperatures of 900–1500 °C and a stepwise one (Ea = 155.0 ± 7.8 kJ mol–1) at higher temperatures of 2100–2400 °C. The secondary gas-phase reactions occurring in the Cat-CVD reactor environment were found to stem from two competing processes. The first one, free-radical short-chain reactions initiated by •CH3 formation and propagated by H abstraction reactions, is the dominating chemical process, producing many high-mass stable alkyl-substituted or silyl-substituted disilazane or trisilazane products via radical recombination reactions. Head-to-tail cycloaddition of unstable DMSA is the second contributing chemical process, which forms cyclodisilazane species. In addition, evidence was found for the conversion of NH3 into H2 and N2 in the Cat-CVD reactor.
中文翻译:

了解 1,1,3,3-四甲基二硅氮烷作为催化化学气相沉积过程中的前体气体的反应化学
催化化学气相沉积 (Cat-CVD) 中 1,1,3,3-四甲基二硅氮烷 (TMDSZ) 的反应化学,包括其在加热的钨丝上的初级分解和 Cat-CVD 反应器中的次级气相反应,使用 10.5 eV 真空紫外单光子电离和/或激光诱导电子电离与飞行时间质谱联用进行研究。已证明TMDSZ最初分解形成各种物质,包括甲基自由基(·CH 3 )、氨(NH 3 )和1,1-二甲基硅胺(DMSA)。在1400-2000 和900-2400 °C 的温度范围内,形成•CH 3和NH 3的活化能( E a )分别为61.2 ± 1.0 和42.1 ± 0.9 kJ mol –1 。研究发现,DMSA 的形成可能有两种不同的贡献途径,即在 900-1500 °C 较低温度下的协同途径(E a = 33.6 ± 2.3 kJ mol –1)和逐步途径(E a = 155.0 ± 7.8 kJ mol –1 ) 在 2100–2400 °C 的较高温度下。人们发现 Cat-CVD 反应器环境中发生的二次气相反应源于两个相互竞争的过程。第一个是由•CH 3形成引发并通过H 夺取反应传播的自由基短链反应,是主要的化学过程,通过自由基重组产生许多高质量稳定的烷基取代或甲硅烷基取代的二硅氮烷或三硅氮烷产物反应。不稳定 DMSA 的头尾环加成是第二个起作用的化学过程,它形成环二硅氮烷物质。此外,还发现了在Cat-CVD反应器中NH 3转化为H 2和N 2的证据。
更新日期:2023-10-26
中文翻译:

了解 1,1,3,3-四甲基二硅氮烷作为催化化学气相沉积过程中的前体气体的反应化学
催化化学气相沉积 (Cat-CVD) 中 1,1,3,3-四甲基二硅氮烷 (TMDSZ) 的反应化学,包括其在加热的钨丝上的初级分解和 Cat-CVD 反应器中的次级气相反应,使用 10.5 eV 真空紫外单光子电离和/或激光诱导电子电离与飞行时间质谱联用进行研究。已证明TMDSZ最初分解形成各种物质,包括甲基自由基(·CH 3 )、氨(NH 3 )和1,1-二甲基硅胺(DMSA)。在1400-2000 和900-2400 °C 的温度范围内,形成•CH 3和NH 3的活化能( E a )分别为61.2 ± 1.0 和42.1 ± 0.9 kJ mol –1 。研究发现,DMSA 的形成可能有两种不同的贡献途径,即在 900-1500 °C 较低温度下的协同途径(E a = 33.6 ± 2.3 kJ mol –1)和逐步途径(E a = 155.0 ± 7.8 kJ mol –1 ) 在 2100–2400 °C 的较高温度下。人们发现 Cat-CVD 反应器环境中发生的二次气相反应源于两个相互竞争的过程。第一个是由•CH 3形成引发并通过H 夺取反应传播的自由基短链反应,是主要的化学过程,通过自由基重组产生许多高质量稳定的烷基取代或甲硅烷基取代的二硅氮烷或三硅氮烷产物反应。不稳定 DMSA 的头尾环加成是第二个起作用的化学过程,它形成环二硅氮烷物质。此外,还发现了在Cat-CVD反应器中NH 3转化为H 2和N 2的证据。






























 京公网安备 11010802027423号
京公网安备 11010802027423号