Journal of Saudi Chemical Society ( IF 5.8 ) Pub Date : 2023-10-27 , DOI: 10.1016/j.jscs.2023.101761 Liangxin Fan , Fangyu He , Lijun Shi , Guoyu Yang , Zhenliang Pan , Miaomiao Wang , Caixia Wang , Lulu Wu , Senyu Han , Yifang Guo , Cuilian Xu
|
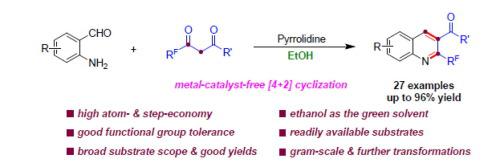
The skeleton of 2-trifluoromethyl quinoline is the core structure of many natural products and pharmaceutical molecules. The synthesis of this scaffold is limited by metal catalysis, harsh conditions, toxic or hazardous reagents and solvents, and lower atom-economy. Herein, a novel method for the synthesis of 2-trifluoromethyl quinolines via the [4 + 2] cyclization of β-keto esters or 1,3-diketones with various substituted o-aminobenzaldehydes using the metal free catalyst in EtOH is reported. This atom- and step-economical protocol features simple operation, broad substrate scope, and good functional-group compatibility. The synthetic utility of this methodology was highlighted by easy gram-scale synthesis and late-stage functionalization, which would promote the vigorous development of quinoline chemistry. Moreover, the in vitro antifungal activities of the 2-trifluoromethyl quinolines against F. graminearum (from wheat), F. graminearum (from corn), F. moniliforme, F. oxysporum, and R. solani were investigated to further potential utility of these compounds.
中文翻译:

无金属条件下2-三氟甲基喹啉衍生物的绿色合成及其抗真菌性能
2-三氟甲基喹啉的骨架是许多天然产物和药物分子的核心结构。该支架的合成受到金属催化、苛刻条件、有毒或有害试剂和溶剂以及较低原子经济性的限制。本文报道了一种在乙醇中使用无金属催化剂,通过β-酮酯或 1,3-二酮与各种取代的邻氨基苯甲醛的 [4 + 2] 环化合成 2-三氟甲基喹啉的新方法。这种原子和步骤经济的方案具有操作简单、底物范围广、官能团兼容性好的特点。该方法的合成实用性突出在于易于克级合成和后期功能化,这将促进喹啉化学的蓬勃发展。此外,还研究了2-三氟甲基喹啉对禾谷镰刀菌(小麦)、禾谷镰刀菌(玉米)、串珠镰刀菌、尖孢镰刀菌和立枯病菌的体外抗真菌活性,以进一步研究这些化合物的潜在用途。化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号