Molecular Catalysis ( IF 3.9 ) Pub Date : 2023-10-26 , DOI: 10.1016/j.mcat.2023.113619
Huibin Li , Yinzhi Pan , Lei Wu , Rui He , Zirong Qin , Shasha Luo , Lijun Yang , Jianhuang Zeng
|
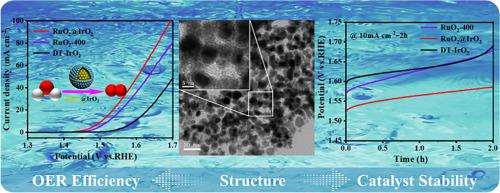
The ever-rising energy demand and environmental concerns require the application of electrochemical water splitting to produce hydrogen as a substitute energy to fossil fuel. Proton exchange membrane water electrolyzers split water into oxygen and hydrogen at the anode and cathode, respectively. Anode catalysts necessitate extensive study, because significant overpotential is required to accelerate the 4-e sluggish oxygen evolution reaction in acidic media. RuO2@IrO2 was synthesized in this work, which aims to achieve high activity and stability. In doing so, RuO2·xH2O was first prepared using the Adams method, followed by the subsequent precipitation of Ir(OH)3 on its surface via the hydrolysis of iridium precursor in a mix of NH3·H2O in ethanol. Upon heat treatment, RuO2@IrO2 with an appropriate Ru/Ir molar ratio was obtained and applied for oxygen evolution reaction. Electrochemical evaluation results show that the optimized RuO2@IrO2-20 performed the best in terms of enhanced mass activity (1.62 A mg−1oxide @1.6 V) and lower overpotential (275 mV@10 mA cm−2) compared with single RuO2 and IrO2 catalysts. In addition, both chronopotentiometry and chronoamperometry test results show that the stability of RuO2@IrO2-20 is highly competitive. The intimate contact between RuO2 and IrO2 combines both the most active RuO2 and stable IrO2 as an integrated catalyst.
中文翻译:

IrO2 沉积在 RuO2 上,形成核壳结构 RuO2@IrO2,用于电化学水电解槽中的析氧反应
不断增长的能源需求和环境问题需要应用电化学水分解来生产氢气作为化石燃料的替代能源。质子交换膜水电解器分别在阳极和阴极将水分解为氧气和氢气。阳极催化剂需要进行广泛的研究,因为需要显着的超电势来加速酸性介质中缓慢的 4-e 析氧反应。本工作合成了RuO 2 @IrO 2,旨在实现高活性和稳定性。在此过程中,首先使用 Adams 方法制备了RuO 2 ·xH 2 O,然后通过在 NH 3 ·H 2 O 与乙醇的混合物中水解铱前体,在其表面沉淀Ir(OH) 3。经过热处理,得到具有适当Ru/Ir摩尔比的RuO 2 @IrO 2 ,并将其用于析氧反应。电化学评价结果表明,与单一材料相比,优化后的RuO 2 @IrO 2 -20在增强的质量活性(1.62 A mg -1氧化物@1.6 V)和较低的过电势(275 mV@10 mA cm -2 )方面表现最好。 RuO 2和IrO 2催化剂。此外,计时电位法和计时电流法测试结果均表明RuO 2 @IrO 2 -20的稳定性具有很强的竞争力。RuO 2和IrO 2之间的紧密接触将最活泼的RuO 2和稳定的IrO 2结合在一起作为整体催化剂。







































 京公网安备 11010802027423号
京公网安备 11010802027423号