当前位置:
X-MOL 学术
›
Gastroenterology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tertiary Lymphoid Structure-Associated B Cells Enhance CXCL13+CD103+CD8+ Tissue-Resident Memory T-Cell Response to Programmed Cell Death Protein 1 Blockade in Cancer Immunotherapy
Gastroenterology ( IF 25.7 ) Pub Date : 2023-10-29 , DOI: 10.1053/j.gastro.2023.10.022
Chupeng Hu 1 , Wenhua You 1 , Deyuan Kong 1 , Yedi Huang 1 , JinYing Lu 1 , Mengya Zhao 1 , Yu Jin 1 , Rui Peng 2 , Dong Hua 3 , Dong-Ming Kuang 4 , Yun Chen 1
Gastroenterology ( IF 25.7 ) Pub Date : 2023-10-29 , DOI: 10.1053/j.gastro.2023.10.022
Chupeng Hu 1 , Wenhua You 1 , Deyuan Kong 1 , Yedi Huang 1 , JinYing Lu 1 , Mengya Zhao 1 , Yu Jin 1 , Rui Peng 2 , Dong Hua 3 , Dong-Ming Kuang 4 , Yun Chen 1
Affiliation
|
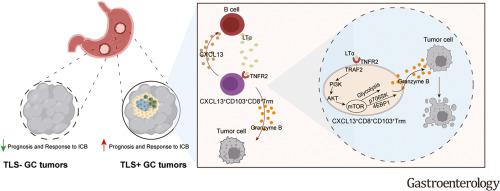
Although the presence of tertiary lymphoid structures (TLS) correlates with positive responses to immunotherapy in many solid malignancies, the mechanism by which TLS enhances antitumor immunity is not well understood. The present study aimed to investigate the underlying cross talk circuits between B cells and tissue-resident memory T (Trm) cells within the TLS and to understand their role in the context of immunotherapy. Immunostaining and H&E staining of TLS and chemokine (C-X-C motif) ligand 13 (CXCL13) cluster of differentiation (CD)103CD8 Trm cells were performed on tumor sections from patients with gastric cancer (GC). The mechanism of communication between B cells and CXCL13CD103CD8 Trm cells was determined in vitro and in vivo. The effect of CXCL13CD103CD8 Trm cells in suppressing tumor growth was evaluated through anti–programmed cell death protein (PD)-1 therapy. The presence of TLS and CXCL13CD103CD8 Trm cells in tumor tissues favored a superior response to anti–PD-1 therapy in patients with GC. Additionally, our research identified that activated B cells enhanced CXCL13 and granzyme B secretion by CD103CD8 Trm cells. Mechanistically, B cells facilitated the glycolysis of CD103CD8 Trm cells through the lymphotoxin-α/tumor necrosis factor receptor 2 (TNFR2) axis, and the mechanistic target of rapamycin signaling pathway played a critical role in CD103CD8 Trm cells glycolysis during this process. Moreover, the presence of TLS and CXCL13CD103CD8 Trm cells correlated with potent responsiveness to anti–PD-1 therapy in a TNFR2-dependent manner. This study further reveals a crucial role for cellular communication between TLS-associated B cell and CXCL13CD103CD8 Trm cells in antitumor immunity, providing valuable insights into the potential use of the lymphotoxin-α/TNFR2 axis within CXCL13CD103CD8 Trm cells for advancing immunotherapy strategies in GC.
中文翻译:

在癌症免疫治疗中,三级淋巴结构相关 B 细胞增强 CXCL13+CD103+CD8+ 组织驻留记忆 T 细胞对程序性细胞死亡蛋白 1 阻断的反应
尽管三级淋巴结构 (TLS) 的存在与许多实体恶性肿瘤对免疫治疗的阳性反应相关,但 TLS 增强抗肿瘤免疫的机制尚不清楚。本研究旨在研究 TLS 内 B 细胞和组织驻留记忆 T (Trm) 细胞之间的潜在串扰回路,并了解它们在免疫治疗中的作用。对胃癌 (GC) 患者的肿瘤切片进行 TLS 和趋化因子 (C-X-C 基序) 配体 13 (CXCL13) 分化簇 (CD)103CD8 Trm 细胞的免疫染色和 H&E 染色。B 细胞和 CXCL13CD103CD8 Trm 细胞之间的通讯机制在体外和体内测定。通过抗程序性细胞死亡蛋白 (PD)-1 疗法评价 CXCL13CD103CD8 Trm 细胞抑制肿瘤生长的效果。肿瘤组织中 TLS 和 CXCL13CD103CD8 Trm 细胞的存在有利于 GC 患者对抗 PD-1 治疗的良好反应。此外,我们的研究发现,活化的 B 细胞增强了 CD103CD8 Trm 细胞的 CXCL13 和颗粒酶 B 分泌。从机制上讲,B 细胞通过淋巴毒素α/肿瘤坏死因子受体 2 (TNFR2) 轴促进 CD103CD8 Trm 细胞的糖酵解,雷帕霉素信号通路的机制靶点在此过程中在 CD103CD8 Trm 细胞糖酵解中起关键作用。此外,TLS 和 CXCL13CD103CD8 Trm 细胞的存在与以 TNFR2 依赖性方式对抗 PD-1 治疗的有效反应相关。 本研究进一步揭示了 TLS 相关 B 细胞和 CXCL13CD103CD8 Trm 细胞之间的细胞通讯在抗肿瘤免疫中的关键作用,为淋巴毒素-α/TNFR2 轴在 CXCL13CD103CD8 Trm 细胞内的潜在用途提供有价值的见解,以推进 GC 中的免疫治疗策略。
更新日期:2023-10-29
中文翻译:

在癌症免疫治疗中,三级淋巴结构相关 B 细胞增强 CXCL13+CD103+CD8+ 组织驻留记忆 T 细胞对程序性细胞死亡蛋白 1 阻断的反应
尽管三级淋巴结构 (TLS) 的存在与许多实体恶性肿瘤对免疫治疗的阳性反应相关,但 TLS 增强抗肿瘤免疫的机制尚不清楚。本研究旨在研究 TLS 内 B 细胞和组织驻留记忆 T (Trm) 细胞之间的潜在串扰回路,并了解它们在免疫治疗中的作用。对胃癌 (GC) 患者的肿瘤切片进行 TLS 和趋化因子 (C-X-C 基序) 配体 13 (CXCL13) 分化簇 (CD)103CD8 Trm 细胞的免疫染色和 H&E 染色。B 细胞和 CXCL13CD103CD8 Trm 细胞之间的通讯机制在体外和体内测定。通过抗程序性细胞死亡蛋白 (PD)-1 疗法评价 CXCL13CD103CD8 Trm 细胞抑制肿瘤生长的效果。肿瘤组织中 TLS 和 CXCL13CD103CD8 Trm 细胞的存在有利于 GC 患者对抗 PD-1 治疗的良好反应。此外,我们的研究发现,活化的 B 细胞增强了 CD103CD8 Trm 细胞的 CXCL13 和颗粒酶 B 分泌。从机制上讲,B 细胞通过淋巴毒素α/肿瘤坏死因子受体 2 (TNFR2) 轴促进 CD103CD8 Trm 细胞的糖酵解,雷帕霉素信号通路的机制靶点在此过程中在 CD103CD8 Trm 细胞糖酵解中起关键作用。此外,TLS 和 CXCL13CD103CD8 Trm 细胞的存在与以 TNFR2 依赖性方式对抗 PD-1 治疗的有效反应相关。 本研究进一步揭示了 TLS 相关 B 细胞和 CXCL13CD103CD8 Trm 细胞之间的细胞通讯在抗肿瘤免疫中的关键作用,为淋巴毒素-α/TNFR2 轴在 CXCL13CD103CD8 Trm 细胞内的潜在用途提供有价值的见解,以推进 GC 中的免疫治疗策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号