Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ubiquitin-specific protease 14 modulates degradation of cellular prion protein.
Scientific Reports ( IF 3.8 ) Pub Date : 2015-Jun-10 , DOI: 10.1038/srep11028
Takujiro Homma , Daisuke Ishibashi , Takehiro Nakagaki , Takayuki Fuse , Tsuyoshi Mori , Katsuya Satoh , Ryuichiro Atarashi , Noriyuki Nishida
Scientific Reports ( IF 3.8 ) Pub Date : 2015-Jun-10 , DOI: 10.1038/srep11028
Takujiro Homma , Daisuke Ishibashi , Takehiro Nakagaki , Takayuki Fuse , Tsuyoshi Mori , Katsuya Satoh , Ryuichiro Atarashi , Noriyuki Nishida
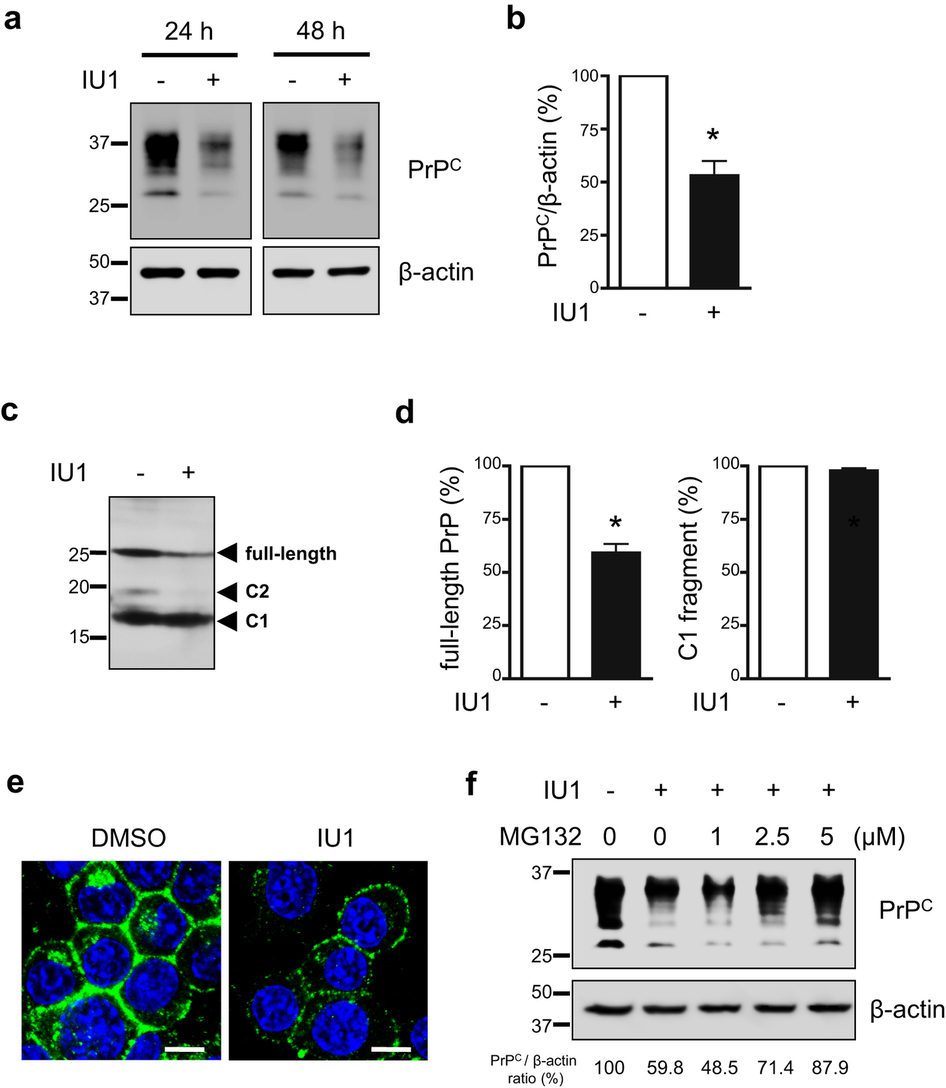
|
Prion diseases are fatal neurodegenerative disorders characterized by the accumulation of prion protein (PrP(C)). To date, there is no effective treatment for the disease. The accumulated PrP, termed PrP(Sc), forms amyloid fibrils and could be infectious. It has been suggested that PrP(Sc) is abnormally folded and resistant to proteolytic degradation, and also inhibits proteasomal functions in infected cells, thereby inducing neuronal death. Recent work indicates that the ubiquitin-proteasome system is involved in quality control of PrP(C). To reveal the significance of prion protein ubiqitination, we focused on ubiquitin-specific protease 14 (USP14), a deubiqutinating enzyme that catalyzes trimming of polyubiquitin chains and plays a role in regulation of proteasomal processes. Results from the present study showed that treatment with a selective inhibitor of USP14 reduced PrP(C), as well as PrP(Sc), levels in prion-infected neuronal cells. Overexpression of the dominant negative mutant form of USP14 reduced PrP(Sc), whereas wildtype USP14 increased PrP(Sc) in prion-infected cells. These results suggest that USP14 prevents degradation of both normal and abnormal PrP. Collectively, a better understanding about the regulation of PrP(Sc) clearance caused by USP14 might contribute greatly to the development of therapeutic strategies for prion diseases.
中文翻译:

泛素特异性蛋白酶14调节细胞蛋白的降解。
on病毒疾病是致命的神经退行性疾病,其特征是of蛋白(PrP(C))的积累。迄今为止,尚无对该疾病的有效治疗方法。积累的PrP(称为PrP(Sc))会形成淀粉样原纤维,并且可能具有传染性。已经提出,PrP(Sc)被异常折叠并且对蛋白水解降解具有抗性,并且还抑制感染细胞中的蛋白酶体功能,从而诱导神经元死亡。最近的工作表明泛素-蛋白酶体系统参与PrP(C)的质量控制。为了揭示病毒蛋白泛素化的重要性,我们集中于泛素特异性蛋白酶14(USP14),这是一种去泛素化酶,可催化多泛素链的修整并在蛋白酶体过程的调节中发挥作用。本研究的结果表明,用USP14的选择性抑制剂治疗可降低感染病毒的神经细胞中的PrP(C)和PrP(Sc)水平。USP14的显性负突变体形式的过表达减少了PrP(Sc),而野生型USP14在病毒感染的细胞中增加了PrP(Sc)。这些结果表明,USP14可防止正常和异常PrP降解。总体而言,更好地了解由USP14引起的PrP(Sc)清除的调控可能会极大地促进病毒疾病的治疗策略的发展。这些结果表明,USP14可防止正常和异常PrP降解。总体而言,更好地了解由USP14引起的PrP(Sc)清除的调控可能会极大地促进病毒疾病的治疗策略的发展。这些结果表明,USP14可防止正常和异常PrP降解。总体而言,更好地了解由USP14引起的PrP(Sc)清除的调控可能会极大地促进病毒疾病的治疗策略的发展。
更新日期:2015-06-12
中文翻译:

泛素特异性蛋白酶14调节细胞蛋白的降解。
on病毒疾病是致命的神经退行性疾病,其特征是of蛋白(PrP(C))的积累。迄今为止,尚无对该疾病的有效治疗方法。积累的PrP(称为PrP(Sc))会形成淀粉样原纤维,并且可能具有传染性。已经提出,PrP(Sc)被异常折叠并且对蛋白水解降解具有抗性,并且还抑制感染细胞中的蛋白酶体功能,从而诱导神经元死亡。最近的工作表明泛素-蛋白酶体系统参与PrP(C)的质量控制。为了揭示病毒蛋白泛素化的重要性,我们集中于泛素特异性蛋白酶14(USP14),这是一种去泛素化酶,可催化多泛素链的修整并在蛋白酶体过程的调节中发挥作用。本研究的结果表明,用USP14的选择性抑制剂治疗可降低感染病毒的神经细胞中的PrP(C)和PrP(Sc)水平。USP14的显性负突变体形式的过表达减少了PrP(Sc),而野生型USP14在病毒感染的细胞中增加了PrP(Sc)。这些结果表明,USP14可防止正常和异常PrP降解。总体而言,更好地了解由USP14引起的PrP(Sc)清除的调控可能会极大地促进病毒疾病的治疗策略的发展。这些结果表明,USP14可防止正常和异常PrP降解。总体而言,更好地了解由USP14引起的PrP(Sc)清除的调控可能会极大地促进病毒疾病的治疗策略的发展。这些结果表明,USP14可防止正常和异常PrP降解。总体而言,更好地了解由USP14引起的PrP(Sc)清除的调控可能会极大地促进病毒疾病的治疗策略的发展。

































 京公网安备 11010802027423号
京公网安备 11010802027423号