当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis Acid and (Hypo)iodite Relay Catalysis Allows a Strategy for the Synthesis of Polysubstituted Azetidines and Tetrahydroquinolines
Organic Letters ( IF 4.9 ) Pub Date : 2016-10-10 00:00:00 , DOI: 10.1021/acs.orglett.6b02430
Jing-Qiang Han 1 , Huan-Huan Zhang 1 , Peng-Fei Xu 1 , Yong-Chun Luo 1
Organic Letters ( IF 4.9 ) Pub Date : 2016-10-10 00:00:00 , DOI: 10.1021/acs.orglett.6b02430
Jing-Qiang Han 1 , Huan-Huan Zhang 1 , Peng-Fei Xu 1 , Yong-Chun Luo 1
Affiliation
|
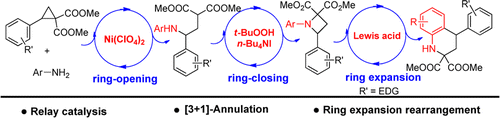
A catalytic [3 + 1]-annulation reaction between cyclopropane 1,1-diester and aromatic amine is developed based on the relay catalysis strategy. Lewis acid-catalyzed nucleophilic ring opening of cyclopropane 1,1-diester with aromatic amine and (hypo)iodite-catalyzed C–N bond formation are combined successfully in one reaction. Using this reaction, biologically important azetidines and tetrahydroquinolines can be prepared directly.
中文翻译:

刘易斯酸和(亚磷酸)碘代中继催化器为多取代的氮杂环丁烷和四氢喹啉的合成提供了一种策略
基于中继催化策略,开发了环丙烷1,1-二酯与芳香胺的催化[3 +1]环化反应。Lewis酸与芳香族胺催化的环丙烷1,1-二酯亲核开环与(次)碘化物催化的C–N键形成成功地结合在一个反应中。使用该反应,可以直接制备生物学上重要的氮杂环丁烷和四氢喹啉。
更新日期:2016-10-10
中文翻译:

刘易斯酸和(亚磷酸)碘代中继催化器为多取代的氮杂环丁烷和四氢喹啉的合成提供了一种策略
基于中继催化策略,开发了环丙烷1,1-二酯与芳香胺的催化[3 +1]环化反应。Lewis酸与芳香族胺催化的环丙烷1,1-二酯亲核开环与(次)碘化物催化的C–N键形成成功地结合在一个反应中。使用该反应,可以直接制备生物学上重要的氮杂环丁烷和四氢喹啉。

































 京公网安备 11010802027423号
京公网安备 11010802027423号