Journal of Molecular Spectroscopy ( IF 1.4 ) Pub Date : 2023-10-27 , DOI: 10.1016/j.jms.2023.111847 Nathan A. Seifert , Branko Ruscic , Raghu Sivaramakrishnan , Kirill Prozument
|
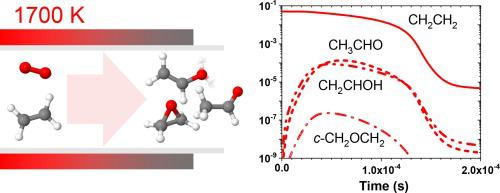
A combined rotational spectroscopy, thermochemistry, and kinetic modeling study explores the mechanism of acetaldehyde (ethanal), vinyl alcohol (ethenol), and ethylene oxide (oxirane) formation in the oxidation of ethylene (ethene). Multiplexed quantitative detection of oxidation intermediates and products exiting a SiO2/SiC microreactor at 1700 K is demonstrated with the BrightSpec W-band chirped-pulse Fourier transform millimeter-wave spectrometer. The broadband rotational spectrum contains transitions of formaldehyde (CH2O), methoxy (CH3O), methanol (CH3OH), ketene (CH2CO), acetaldehyde (CH3CHO), syn-vinyl alcohol (syn-CH2CHOH), anti-vinyl alcohol (anti-CH2CHOH), oxirane (c-CH2OCH2), propyne (CH3CCH), and syn-propanal (syn-CH3CH2CHO). We focus on the three C2H4O species and deduce their concentration ratio [CH3CHO]:[CH2CHOH]:[c-CH2OCH2] = 1:0.7(2):0.06(2) by comparing the observed line intensities to those simulated with the PGOPHER software. Detailed thermochemistry of the C2H4O isomers and conformers is provided by Active Thermochemical Tables. The observed excess concentrations of vinyl alcohol and oxirane relative to the more stable acetaldehyde compared to the equilibrium ratio at 1700 K (1:0.087:0.000024), point to direct chemical pathways to these higher energy isomers. The mechanism for the formation of the three C2H4O isomers is analyzed using a 0-D homogenous reactor kinetics simulation for ethylene oxidation. The ratios of the C2H4O isomers concentrations predicted by the kinetic model are compared to the experimental values.
中文翻译:

乙烯氧化中的C2H4O异构体
旋转光谱、热化学和动力学建模相结合的研究探索了乙烯 (ethene) 氧化过程中乙醛 (ethanal)、乙烯醇 (ethenol) 和环氧乙烷 (oxirane) 形成的机制。使用 BrightSpec W 波段啁啾脉冲傅里叶变换毫米波光谱仪演示了在 1700 K 下对SiO 2 /SiC 微反应器中排出的氧化中间体和产物进行多重定量检测。宽带旋转光谱包含甲醛 (CH 2 O)、甲氧基 (CH 3 O)、甲醇 (CH 3 OH)、乙烯酮 (CH 2 CO)、乙醛 (CH 3 CHO)、顺式乙烯醇 ( syn -CH)的跃迁2 CHOH)、反乙烯醇(反-CH 2 CHOH)、环氧乙烷( c -CH 2 OCH 2 )、丙炔(CH 3 CCH)和顺式丙醛( syn -CH 3 CH 2 CHO)。我们重点关注三种C 2 H 4 O物种,通过比较推导出它们的浓度比[CH 3 CHO]:[CH 2 CHOH]:[ c -CH 2 OCH 2 ] = 1:0.7(2):0.06(2)观察到的线强度与使用 PGOPHER 软件模拟的线强度的比较。C 2 H 4 O 异构体和构象异构体的详细热化学由活性热化学表提供。与 1700 K 下的平衡比 (1:0.087:0.000024) 相比,观察到相对于更稳定的乙醛,乙烯醇和环氧乙烷的浓度过量,表明存在这些更高能量异构体的直接化学途径。使用乙烯氧化的0-D均相反应器动力学模拟分析了三种C 2 H 4 O异构体的形成机理。将动力学模型预测的C 2 H 4 O异构体浓度比与实验值进行比较。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号