当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total synthesis of (S)-forphenicinol via asymmetric organocatalysis
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2023-10-27 , DOI: 10.1039/d3nj04527g R. A. Kovalevsky 1, 2 , A. S. Kucherenko 1 , S. G. Zlotin 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2023-10-27 , DOI: 10.1039/d3nj04527g R. A. Kovalevsky 1, 2 , A. S. Kucherenko 1 , S. G. Zlotin 1
Affiliation
|
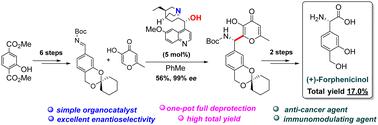
Practical asymmetric synthesis of (S)-forphenicinol, the active ingredient of immunomodulator and anticancer drug Forfenimex®, from commercially available 2-hydroxy-dimethylterephthalate has been developed. Key steps of the synthesis are a stereogenic-center-forming enantioselective organocatalytic Mannich reaction of protected arylcarbaldimine with a kojic acid derivative and oxidative transformation of the γ-pyrone fragment into the carboxylic group. The total yield of (S)-forphenicinol hydrochloride (99% ee) via the proposed nine-step synthetic scheme (17%) is significantly higher than those attained by known methods (4.6% and 0.8%).
中文翻译:

不对称有机催化全合成(S)-forphenicinol
已经开发出以市售的 2-羟基对苯二甲酸二甲酯为原料,实用不对称合成 ( S )-forphenicinol(免疫调节剂和抗癌药物 Forfenimex® 的活性成分)的方法。合成的关键步骤是受保护的芳基卡巴亚胺与曲酸衍生物的立体中心形成对映选择性有机催化曼尼希反应以及γ-吡喃酮片段氧化转化为羧基。通过所提出的九步合成方案( S)-盐酸福苯尼酚(99%ee)的总产率(17%)显着高于通过已知方法获得的总产率(4.6%和0.8%)。
更新日期:2023-11-01
中文翻译:

不对称有机催化全合成(S)-forphenicinol
已经开发出以市售的 2-羟基对苯二甲酸二甲酯为原料,实用不对称合成 ( S )-forphenicinol(免疫调节剂和抗癌药物 Forfenimex® 的活性成分)的方法。合成的关键步骤是受保护的芳基卡巴亚胺与曲酸衍生物的立体中心形成对映选择性有机催化曼尼希反应以及γ-吡喃酮片段氧化转化为羧基。通过所提出的九步合成方案( S)-盐酸福苯尼酚(99%ee)的总产率(17%)显着高于通过已知方法获得的总产率(4.6%和0.8%)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号