当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trifluoromethylarylation of alkenes using anilines
Chemical Science ( IF 7.6 ) Pub Date : 2023-10-27 , DOI: 10.1039/d3sc03868h Carlos Corral Suarez 1, 2 , Ignacio Colomer 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2023-10-27 , DOI: 10.1039/d3sc03868h Carlos Corral Suarez 1, 2 , Ignacio Colomer 1, 2
Affiliation
|
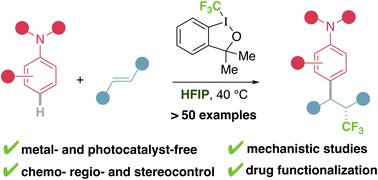
Nitrogen containing compounds, such as anilines, are some of the most widespread and useful chemical species, although their high and unselective reactivity has prevented their incorporation into many interesting transformations, such as the functionalization of alkenes. Herein we report a method that allows the trifluoromethylarylation of alkenes using anilines, for the first time, with no need for additives, transition metals, photocatalysts or an excess of reagents. An in-depth mechanistic study reveals the key role of hexafluoroisopropanol (HFIP) as a unique solvent, establishing a hydrogen bonding network with aniline and trifluoromethyl reagent, that is responsible for the altered reactivity and exquisite selectivity. This work uncovers a new mode of reactivity that involves the use of abundant anilines as a non-prefunctionalized aromatic source and the simultaneous activation of trifluoromethyl hypervalent iodine reagent.
中文翻译:

使用苯胺对烯烃进行三氟甲基芳基化
含氮化合物(例如苯胺)是最广泛和最有用的化学物质之一,尽管它们的高且非选择性反应性阻碍了它们并入许多有趣的转化,例如烯烃的官能化。在此,我们首次报道了一种使用苯胺对烯烃进行三氟甲基芳基化的方法,无需添加剂、过渡金属、光催化剂或过量试剂。深入的机理研究揭示了六氟异丙醇 (HFIP) 作为一种独特溶剂的关键作用,与苯胺和三氟甲基试剂建立氢键网络,从而改变了反应活性和精湛的选择性。这项工作揭示了一种新的反应模式,涉及使用丰富的苯胺作为非预官能化芳香族源,并同时激活三氟甲基高价碘试剂。
更新日期:2023-10-27
中文翻译:

使用苯胺对烯烃进行三氟甲基芳基化
含氮化合物(例如苯胺)是最广泛和最有用的化学物质之一,尽管它们的高且非选择性反应性阻碍了它们并入许多有趣的转化,例如烯烃的官能化。在此,我们首次报道了一种使用苯胺对烯烃进行三氟甲基芳基化的方法,无需添加剂、过渡金属、光催化剂或过量试剂。深入的机理研究揭示了六氟异丙醇 (HFIP) 作为一种独特溶剂的关键作用,与苯胺和三氟甲基试剂建立氢键网络,从而改变了反应活性和精湛的选择性。这项工作揭示了一种新的反应模式,涉及使用丰富的苯胺作为非预官能化芳香族源,并同时激活三氟甲基高价碘试剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号