Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-10-26 , DOI: 10.1016/j.actbio.2023.10.024
Zhipeng Yao 1 , Chenxue Qi 2 , Fan Zhang 3 , Hong Yao 4 , Cheng Wang 5 , Xiaoxiang Cao 6 , Chenhui Zhao 6 , Zhichun Wang 6 , Min Qi 6 , Chengyun Yao 7 , Xiaoming Wang 8 , Hongping Xia 9
|
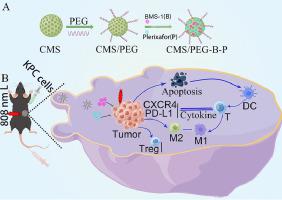
Pancreatic ductal adenocarcinoma (PDAC) is a fatal disease that responds poorly to single-drug immunotherapy with PD-L1 (CD274) inhibitors. Here, we prepared mesoporous nanomaterials Cu2MoS4 (CMS)/PEG loaded with PD-L1 inhibitor BMS-1 and CXCR4 inhibitor Plerixafor to form the nanodrug CMS/PEG-B-P. In vitro experiments, CMS/PEG-B-P have a more substantial inhibitory effect on the expression of PD-L1 and CXCR4 as well as to promote the apoptosis of pancreatic cancer cells KPC and suppressed KPC cell proliferation were detected by flow cytometry, qPCR and Western blotting (WB). Promotes the release of the cytotoxic substance reactive oxygen species (ROS) and the production of the immunogenic cell death (ICD) marker calreticulin (CRT) in KPC cells. CMS/PEG-B-P was also detected to have a certain activating effect on mouse immune cells, dendritic cells (mDC) and macrophage RAW264.7. Subcutaneous tumorigenicity experiments in C57BL/6 mice verified that CMS/PEG-B-P had an inhibitory effect on the growth of tumors and remodeling of the tumor immune microenvironment, including infiltration of CD4+ and CD8+ T cells and polarization of macrophages, as well as reduction of immunosuppressive cells. Meanwhile, CMS/PEG-B-P was found to have different effects on the release of cytokines in the tumor immune microenvironment, including The levels of immunostimulatory cytokines INF-γ and IL-12 are increased and the levels of immunosuppressive cytokines IL-6, IL-10 and IFN-α are decreased. In conclusion, nanomaterial-loaded immune checkpoint inhibitor therapies can enhance the immune response and reduce side effects, a combination that shows great potential as a new immunotherapeutic approach.
Statement of significance
Pancreatic ductal adenocarcinoma (PDAC) is a fatal disease that has a low response to single-drug immunotherapy with PD-L1 (CD274) inhibitors. We preared PEG-modified mesoporous nanomaterials Cu2MoS4 (CMS) loaded with PD-L1 inhibitor BMS-1 and CXCR4 inhibitor Plerixafor to form the nanodrug CMS/PEG-B-P. Our study demonstrated that Nanomaterial-loaded immune checkpoint inhibitor therapies can enhance the immune response and reduce side effects, a combination that shows great potential as a new immunotherapeutic approach.
中文翻译:

负载免疫检查点抑制剂的空心 Cu2MoS4 纳米粒子重塑肿瘤微环境以增强胰腺癌的免疫治疗
胰腺导管腺癌 (PDAC) 是一种致命性疾病,对 PD-L1 (CD274) 抑制剂的单一药物免疫治疗反应不佳。在这里,我们制备了负载PD-L1抑制剂BMS-1和CXCR4抑制剂Plerixafor的介孔纳米材料Cu 2 MoS 4 (CMS)/PEG,形成纳米药物CMS/PEG-BP。体外实验中,流式细胞术、qPCR和Western blot检测发现,CMS/PEG-BP对PD-L1和CXCR4的表达有较明显的抑制作用,并能促进胰腺癌细胞KPC的凋亡,抑制KPC细胞的增殖。印迹(WB)。促进 KPC 细胞中细胞毒性物质活性氧 (ROS) 的释放和免疫原性细胞死亡 (ICD) 标记物钙网蛋白 (CRT) 的产生。还检测到CMS/PEG-BP对小鼠免疫细胞、树突状细胞(mDC)和巨噬细胞RAW264.7有一定的激活作用。 C57BL/6小鼠皮下致瘤实验证实,CMS/PEG-BP对肿瘤生长和肿瘤免疫微环境重塑具有抑制作用,包括CD4 +和CD8 + T细胞的浸润和巨噬细胞的极化,以及免疫抑制细胞减少。同时发现CMS/PEG-BP对肿瘤免疫微环境中细胞因子的释放有不同的影响,包括免疫刺激性细胞因子INF-γ和IL-12水平升高以及免疫抑制性细胞因子IL-6、IL-12水平升高。 -10和IFN-α减少。总之,纳米材料负载的免疫检查点抑制剂疗法可以增强免疫反应并减少副作用,这种组合作为一种新的免疫治疗方法显示出巨大的潜力。
重要性声明
胰腺导管腺癌 (PDAC) 是一种致命性疾病,对 PD-L1 (CD274) 抑制剂的单药免疫疗法反应较低。我们制备了负载PD-L1抑制剂BMS-1和CXCR4抑制剂Plerixafor的PEG修饰介孔纳米材料Cu2MoS4(CMS),形成纳米药物CMS/PEG-BP。我们的研究表明,纳米材料负载的免疫检查点抑制剂疗法可以增强免疫反应并减少副作用,这种组合显示出作为新免疫治疗方法的巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号