Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-10-25 , DOI: 10.1016/j.molstruc.2023.136936 Muhammad Umair , Aziz-ur-Rehman , Muhammad Athar Abbasi , Sabahat Zahra Siddiqui , Javed Iqbal , Shahid Rasool , Shafi Ullah Khan , Syed Adnan Ali Shah
|
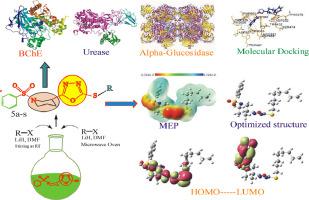
The diverse biological activities of 1,3,4-oxadiazole derivatives are very significant due to structural complexity. Concentrating on metal free synthesis of 1,3,4-oxadiazole hybrids, 5a-s were obtained by two methodologies including conventional and microwave assisted. The microwave assisted synthesis was proved to be better option for selection in future due to time saving and excellent yield. 1,3,4-Oxadiazole, 3, was synthesized in three consecutive phases and finally reacted with a series of electrophiles, 4a-s, acknowledged as alkyl halide to get the anticipated compounds. Final compounds were acquired through two different routes including conventional and microwave assisted methods. IR, 1H-NMR, 13C-NMR and elemental analysis were performed for the structural elucidation of synthesized derivatives. B3LYP method and the basis set of 6-311++G (d,p) were used for natural bond orbital and structural optimization. The time-dependent density functional theory (TD-DFT) helped for the calculation of frontier molecular orbitals (FMOs) and molecular electrostatic potential (MEP) at the same level of selected compounds as potential candidates against the enzymes taken into account. The screenings of all the nineteen derivatives were performed against alpha-glucosidase, urease and butyryl cholinesterase enzymes. Six compounds actively inhibited alpha-glucosidase in comparison with acarbose; four actively inhibited urease in comparison with thiourea; and five actively inhibited butyryl cholinesterase in comparison with eserine. Three compounds, 5n, 5a and 5q showed good inhibition potential and found the best in the synthesized series.
中文翻译:

碱金属氢化物催化含吖嗪的恶二唑与取代卤代烷多选择性反应制备抗酶剂
1,3,4-恶二唑衍生物由于结构复杂,其多样化的生物活性非常显着。专注于 1,3,4-恶二唑杂化物的无金属合成,通过常规方法和微波辅助两种方法获得了5a-s 。由于节省时间和优异的产率,微波辅助合成被证明是未来更好的选择。1,3,4-恶二唑, 3 , 分三个连续阶段合成,最后与一系列亲电试剂4a-s(被称为卤代烷)反应得到预期的化合物。最终化合物通过两种不同的途径获得,包括传统方法和微波辅助方法。通过IR、1 H-NMR、13 C-NMR和元素分析对合成衍生物进行了结构解析。采用B3LYP方法和6-311++G(d,p)基组进行自然键轨道和结构优化。时间相关密度泛函理论 (TD-DFT) 有助于在选定化合物的同一水平上计算前沿分子轨道 (FMO) 和分子静电势 (MEP),作为针对所考虑酶的潜在候选化合物。所有十九种衍生物的筛选都是针对α-葡萄糖苷酶、脲酶和丁酰胆碱酯酶进行的。与阿卡波糖相比,六种化合物可有效抑制 α-葡萄糖苷酶;与硫脲相比,四种活性抑制脲酶;与 eserine 相比,有 5 种活性抑制丁酰胆碱酯酶。三种化合物5n、5a和5q显示出良好的抑制潜力,是合成系列中最好的。































 京公网安备 11010802027423号
京公网安备 11010802027423号