当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CHNO – Formylnitrene, Cyanic, Isocyanic, Fulminic, and Isofulminic Acids and their Interrelationships at DFT and CASPT2 Levels of Theory
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-10-24 , DOI: 10.1021/acs.jpca.3c05805 Didier Bégué 1 , William Lafargue-Dit-Hauret 1 , Alain Dargelos 1 , Curt Wentrup 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-10-24 , DOI: 10.1021/acs.jpca.3c05805 Didier Bégué 1 , William Lafargue-Dit-Hauret 1 , Alain Dargelos 1 , Curt Wentrup 2
Affiliation
|
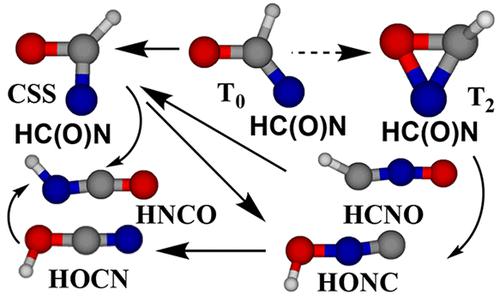
Fulminic and cyanic acids played a decisive role in the conception of isomerism 200 years ago. Cyanic (HOCN), isocyanic (HNCO), and fulminic (HCNO) acids have been detected in several interstellar sources, but isofulminic acid (HONC) is little known. Here we examine the interrelationships between the four acids and formylnitrene, HC(O)N, at the CASPT2 and three DFT levels. Formylnitrene has a triplet ground state, T0, a closed shell singlet (CSS), S0, and an open-shell singlet (OSS), S1, lying ∼7 and 27 kcal/mol above T0, respectively. The CSS is weakly stabilized by a 12 kcal/mol bond between the N and the O atoms. A conical intersection 12 kcal/mol above T0 permits easy T0–S0 interchange. Formyl azide and formylnitrene (T0 and S0) are isomerized thermally to HNCO. HOCN is best obtained via dissociation of the nitrene (or of HNCO) to H• + NCO• radicals ∼46 kcal/mol above the T0 nitrene. Isofulminic acid, HONC, isomerizes readily to cyanic acid, HOCN, in thermal and photochemical reactions. Fulminic acid, HCNO, can isomerize to HNCO via CSS formylnitrene. Easy tautomerization prevents the preparation of HOCN in quantity. The barrier to isomerization is strongly reduced in small hydrogen-bonded aggregates so that trace amounts of HOCN can exist in equilibrium with HNCO.
中文翻译:

CHNO – 甲酰基氮烯、氰酸、异氰酸、雷酸和异雷酸及其在 DFT 和 CASPT2 理论水平上的相互关系
200 年前,雷酸和氰酸在异构现象的概念中发挥了决定性作用。氰酸 (HOCN)、异氰酸 (HNCO) 和雷酸 (HCNO) 已在多个星际来源中检测到,但异雷酸 (HONC) 却鲜为人知。在这里,我们在 CASPT2 和三个 DFT 水平上检查了四种酸和甲酰基氮烯 HC(O)N 之间的相互关系。甲酰氮烯具有三线态基态T 0、闭壳单线态 (CSS) S 0和开壳单线态 (OSS) S 1 ,分别比T 0高约 7 和 27 kcal/mol 。CSS 通过 N 和 O 原子之间的 12 kcal/mol 键进行弱稳定。T 0以上 12 kcal/mol 的圆锥形交叉点可以轻松实现T 0 – S 0互换。甲酰叠氮和甲酰氮烯(T 0和S 0)热异构化为HNCO。HOCN最好通过氮烯(或HNCO)解离为高于T 0氮烯约46 kcal/mol的H • + NCO •自由基来获得。异烟酸 HONC 在热和光化学反应中很容易异构化为氰酸 HOCN。雷米酸 HCNO 可通过 CSS 甲酰基氮烯异构化为 HNCO。容易的互变异构阻止了 HOCN 的大量制备。在小的氢键聚集体中,异构化的障碍大大降低,因此痕量的 HOCN 可以与 HNCO 平衡存在。
更新日期:2023-10-24
中文翻译:

CHNO – 甲酰基氮烯、氰酸、异氰酸、雷酸和异雷酸及其在 DFT 和 CASPT2 理论水平上的相互关系
200 年前,雷酸和氰酸在异构现象的概念中发挥了决定性作用。氰酸 (HOCN)、异氰酸 (HNCO) 和雷酸 (HCNO) 已在多个星际来源中检测到,但异雷酸 (HONC) 却鲜为人知。在这里,我们在 CASPT2 和三个 DFT 水平上检查了四种酸和甲酰基氮烯 HC(O)N 之间的相互关系。甲酰氮烯具有三线态基态T 0、闭壳单线态 (CSS) S 0和开壳单线态 (OSS) S 1 ,分别比T 0高约 7 和 27 kcal/mol 。CSS 通过 N 和 O 原子之间的 12 kcal/mol 键进行弱稳定。T 0以上 12 kcal/mol 的圆锥形交叉点可以轻松实现T 0 – S 0互换。甲酰叠氮和甲酰氮烯(T 0和S 0)热异构化为HNCO。HOCN最好通过氮烯(或HNCO)解离为高于T 0氮烯约46 kcal/mol的H • + NCO •自由基来获得。异烟酸 HONC 在热和光化学反应中很容易异构化为氰酸 HOCN。雷米酸 HCNO 可通过 CSS 甲酰基氮烯异构化为 HNCO。容易的互变异构阻止了 HOCN 的大量制备。在小的氢键聚集体中,异构化的障碍大大降低,因此痕量的 HOCN 可以与 HNCO 平衡存在。






























 京公网安备 11010802027423号
京公网安备 11010802027423号