当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight from Lewis-Acid-Dependent Selectivity and Reversible Haloboration, as Harnessed for Boron-Based Electrophilic Cyclization Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-10-25 , DOI: 10.1021/acs.joc.3c01653 Martin Stang 1 , Robert J Mycka 1, 2 , Suzanne A Blum 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-10-25 , DOI: 10.1021/acs.joc.3c01653 Martin Stang 1 , Robert J Mycka 1, 2 , Suzanne A Blum 1
Affiliation
|
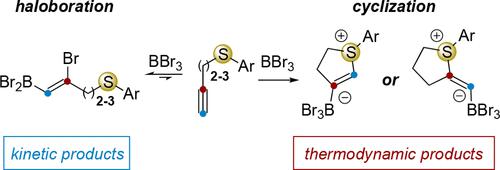
Different reaction selectivity occurs with the Lewis acids B-chlorocatecholborane (ClBcat), B-bromocatecholborane (BrBcat), and BBr3, favoring either alkyne haloboration, electrophilic cyclization of a tethered nucleophilic sulfur onto the alkyne, or group transfer of the nucleophile. This reaction selectivity also depends on the chain length of the tethered nucleophile, revealing a subtle interplay of relative kinetics and thermodynamics. In all cases, BBr3 reacts readily with alkynes to form haloborated products; however, this process is reversible, and this reversibility can be harnessed to ultimately access regio- and stereodefined cyclic sulfonium zwitterions via the slower but thermodynamically favored electrophilic cyclization pathway. Reversibility was noted by following the reaction by NMR spectroscopy, and by characterizing the kinetic and thermodynamic products by a combination of 2D NMR spectroscopy and single-crystal X-ray diffraction. The “mixed” reagent bromocatechol borane (BrBcat) displayed reactivity between ClBcat and BBr3, producing bromoboration in some cases and electrophilic cyclization in others. With this enhanced understanding of the reaction dynamics, it becomes possible to use boron Lewis acids in a predictable manner in cases where haloboration is the kinetic product but in which the reversibility of this reaction maintains access to eventual alternative reactivity leading to desired building blocks in organic synthesis.
中文翻译:

来自路易斯酸依赖性选择性和可逆卤硼化的机理见解,用于硼基亲电环化反应
路易斯酸B-氯儿茶酚硼烷 (ClBcat)、B-溴儿茶酚硼烷 (BrBcat) 和 BBr 3会发生不同的反应选择性,有利于炔烃卤硼化、束缚亲核硫在炔烃上的亲电环化或亲核试剂的基团转移。这种反应选择性还取决于束缚亲核试剂的链长,揭示了相对动力学和热力学的微妙相互作用。在所有情况下,BBr 3都很容易与炔烃反应形成卤硼化产物;然而,这个过程是可逆的,并且可以利用这种可逆性通过较慢但热力学有利的亲电环化途径最终获得区域和立体定义的环状锍两性离子。通过 NMR 光谱跟踪反应,并结合 2D NMR 光谱和单晶 X 射线衍射表征动力学和热力学产物,注意到可逆性。“混合”试剂溴儿茶酚硼烷 (BrBcat) 在 ClBcat 和 BBr 3之间表现出反应性,在某些情况下产生溴硼化反应,在另一些情况下产生亲电环化。随着对反应动力学的加深了解,在卤硼化是动力学产物但该反应的可逆性保持获得最终替代反应性的情况下,可以以可预测的方式使用硼路易斯酸,从而产生所需的有机结构单元。合成。
更新日期:2023-10-25
中文翻译:

来自路易斯酸依赖性选择性和可逆卤硼化的机理见解,用于硼基亲电环化反应
路易斯酸B-氯儿茶酚硼烷 (ClBcat)、B-溴儿茶酚硼烷 (BrBcat) 和 BBr 3会发生不同的反应选择性,有利于炔烃卤硼化、束缚亲核硫在炔烃上的亲电环化或亲核试剂的基团转移。这种反应选择性还取决于束缚亲核试剂的链长,揭示了相对动力学和热力学的微妙相互作用。在所有情况下,BBr 3都很容易与炔烃反应形成卤硼化产物;然而,这个过程是可逆的,并且可以利用这种可逆性通过较慢但热力学有利的亲电环化途径最终获得区域和立体定义的环状锍两性离子。通过 NMR 光谱跟踪反应,并结合 2D NMR 光谱和单晶 X 射线衍射表征动力学和热力学产物,注意到可逆性。“混合”试剂溴儿茶酚硼烷 (BrBcat) 在 ClBcat 和 BBr 3之间表现出反应性,在某些情况下产生溴硼化反应,在另一些情况下产生亲电环化。随着对反应动力学的加深了解,在卤硼化是动力学产物但该反应的可逆性保持获得最终替代反应性的情况下,可以以可预测的方式使用硼路易斯酸,从而产生所需的有机结构单元。合成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号