当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic, asymmetric carbon–nitrogen bond formation using metal nitrenoids: from metal–ligand complexes via metalloporphyrins to enzymes
Chemical Science ( IF 7.6 ) Pub Date : 2023-10-25 , DOI: 10.1039/d3sc04661c Alexander Fanourakis 1 , Robert J Phipps 1
Chemical Science ( IF 7.6 ) Pub Date : 2023-10-25 , DOI: 10.1039/d3sc04661c Alexander Fanourakis 1 , Robert J Phipps 1
Affiliation
|
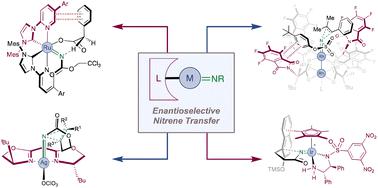
The introduction of nitrogen atoms into small molecules is of fundamental importance and it is vital that ever more efficient and selective methods for achieving this are developed. With this aim, the potential of nitrene chemistry has long been appreciated but its application has been constrained by the extreme reactivity of these labile species. This liability however can be attenuated by complexation with a transition metal and the resulting metal nitrenoids have unique and highly versatile reactivity which includes the amination of certain types of aliphatic C–H bonds as well as reactions with alkenes to afford aziridines. At least one new chiral centre is typically formed in these processes and the development of catalysts to exert control over enantioselectivity in nitrenoid-mediated amination has become a growing area of research, particularly over the past two decades. Compared with some synthetic methods, metal nitrenoid chemistry is notable in that chemists can draw from a diverse array of metals and catalysts , ranging from metal–ligand complexes, bearing a variety of ligand types, via bio-inspired metalloporphyrins, all the way through to, very recently, engineered enzymes themselves. In the latter category in particular, rapid progress is being made, the rate of which suggests that this approach may be instrumental in addressing some of the outstanding challenges in the field. This review covers key developments and strategies that have shaped the field, in addition to the latest advances, up until September 2023.
中文翻译:

使用金属氮烯类催化形成不对称碳氮键:从金属配体络合物通过金属卟啉到酶
将氮原子引入小分子中具有根本性的重要性,并且开发更有效和选择性的方法来实现这一点至关重要。出于这一目标,氮烯化学的潜力长期以来一直受到人们的重视,但其应用一直受到这些不稳定物质的极端反应性的限制。然而,这种责任可以通过与过渡金属络合来减弱,并且所得的金属氮烯类化合物具有独特且高度通用的反应性,其中包括某些类型的脂肪族C-H键的胺化以及与烯烃的反应以提供氮丙啶。在这些过程中通常会形成至少一个新的手性中心,并且开发催化剂来控制氮烯介导的胺化中的对映选择性已成为一个不断增长的研究领域,特别是在过去的二十年中。与一些合成方法相比,金属氮烯化学的显着之处在于,化学家可以从多种金属和催化剂中提取,从带有各种配体类型的金属配体络合物,通过仿生金属卟啉,一直到,最近,酶本身被改造。特别是在后一类中,正在取得快速进展,其速度表明这种方法可能有助于解决该领域的一些突出挑战。除了截至 2023 年 9 月的最新进展外,本次回顾还涵盖了塑造该领域的关键发展和战略。
更新日期:2023-10-25
中文翻译:

使用金属氮烯类催化形成不对称碳氮键:从金属配体络合物通过金属卟啉到酶
将氮原子引入小分子中具有根本性的重要性,并且开发更有效和选择性的方法来实现这一点至关重要。出于这一目标,氮烯化学的潜力长期以来一直受到人们的重视,但其应用一直受到这些不稳定物质的极端反应性的限制。然而,这种责任可以通过与过渡金属络合来减弱,并且所得的金属氮烯类化合物具有独特且高度通用的反应性,其中包括某些类型的脂肪族C-H键的胺化以及与烯烃的反应以提供氮丙啶。在这些过程中通常会形成至少一个新的手性中心,并且开发催化剂来控制氮烯介导的胺化中的对映选择性已成为一个不断增长的研究领域,特别是在过去的二十年中。与一些合成方法相比,金属氮烯化学的显着之处在于,化学家可以从多种金属和催化剂中提取,从带有各种配体类型的金属配体络合物,通过仿生金属卟啉,一直到,最近,酶本身被改造。特别是在后一类中,正在取得快速进展,其速度表明这种方法可能有助于解决该领域的一些突出挑战。除了截至 2023 年 9 月的最新进展外,本次回顾还涵盖了塑造该领域的关键发展和战略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号