Journal of Drug Delivery Science and Technology ( IF 4.5 ) Pub Date : 2023-10-25 , DOI: 10.1016/j.jddst.2023.105081 Sakshi Priya , Gorantla Srividya , Nittala Sarath Chandra , Prem Prakash Singh , Ranendra N. Saha , Priyadarshini Sathe , Jayabalan Nirmal , Gautam Singhvi
|
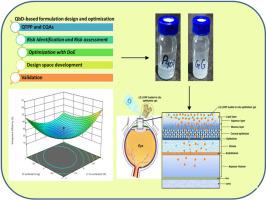
Ophthalmic treatment is challenging due to the numerous physiological barriers present in the eye. Loteprednol Etabonate (LE) is a corticosteroid used to treat ophthalmic inflammation. LE is currently available in the form of ointment, gel, and suspension drops. In this study, LE lyotropic liquid crystalline nanoparticle (LCNP) in-situ ophthalmic gel was formulated to get over the drawbacks of conventional drug delivery systems like low penetration, retention, and bioavailability of the drug through the cornea's surface. The objective of the proposed study was to develop LE-LCNP using a three-factor, five-level Central Composite Design of Response Surface Methodology. The optimized batch of LE-LCNP exhibited a particle size of 102.5 ± 10.19 nm, PDI of 0.319 ± 0.120, and entrapment efficiency of 57.32 ± 3.44%. The in-vitro results revealed 99.99% drug release from LE-LCNP in 18 h. Further, the optimized LE-loaded LCNP formulation was incorporated into in-situ gelling systems to formulate and characterize a temperature-sensitive and ion-activated in-situ ophthalmic gel using Poloxamer 407 (P407) and Gellan gum (GG). The rheological study showed that the viscosity of LE-LCNP P407 and LE-LCNP GG in-situ ophthalmic gel formulation was 4.7 and 3.9-fold higher than marketed LE suspension, respectively at 25 °C. The higher viscous characteristics of the LE-loaded LCNP in-situ ophthalmic gel led to improved ocular retention, efficacy, and patient compliance. The ex-vivo corneal permeation study of LE-LCNP P407 and LE-LCNP GG in-situ ophthalmic gel showed 7 and 2.5-fold higher ocular permeation when compared with the marketed LE suspension, respectively. The results suggested that LE-loaded LCNP in-situ ophthalmic gel has the potential to become an effective carrier system for the treatment of inflammatory disorders of the eyes.
中文翻译:

依碳氯替泼诺负载溶致液晶纳米颗粒原位眼科凝胶:Qbd 驱动的优化和持续角膜前停留时间的体外、离体证据
由于眼睛中存在许多生理障碍,眼科治疗具有挑战性。Loteprednol Etabonate (LE) 是一种皮质类固醇,用于治疗眼部炎症。LE 目前有软膏、凝胶和悬浮滴剂的形式。在这项研究中,配制了 LE 溶致液晶纳米颗粒 (LCNP) 原位眼科凝胶,以克服传统药物递送系统的缺点,例如药物通过角膜表面的渗透性、保留性和生物利用度低。拟议研究的目标是使用响应面方法的三因素、五水平中心复合设计来开发 LE-LCNP。优化后的 LE-LCNP 批次粒径为 102.5 ± 10.19 nm,PDI 为 0.319 ± 0.120,包封率为 57.32 ± 3.44%。体外结果显示 LE-LCNP 在 18 小时内释放了 99.99% 的药物。此外,将优化的 LE 负载 LCNP 配方纳入原位胶凝系统中,使用泊洛沙姆 407 (P407) 和结冷胶 (GG) 配制和表征温度敏感且离子活化的原位眼科凝胶。流变学研究表明,25°C 时,LE-LCNP P407 和 LE-LCNP GG 原位眼用凝胶制剂的粘度分别比市售 LE 悬浮液高 4.7 倍和 3.9 倍。负载 LE 的 LCNP 原位眼科凝胶具有较高的粘度特性,可改善眼部保留、功效和患者依从性。LE-LCNP P407 和 LE-LCNP GG原位眼用凝胶的离体角膜渗透性研究显示,与市售 LE 悬浮液相比,眼部渗透性分别高 7 倍和 2.5 倍。结果表明,负载 LE 的 LCNP 原位眼科凝胶有潜力成为治疗眼部炎症性疾病的有效载体系统。






































 京公网安备 11010802027423号
京公网安备 11010802027423号