当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N‐Substituted Azacalixphyrins: Synthesis, Properties, and Self‐Assembly
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-10-11 , DOI: 10.1002/chem.201602288 Zhongrui Chen 1 , Rose Haddoub 1 , Jérôme Mahé 2 , Gabriel Marchand 2 , Denis Jacquemin 2, 3 , Judicaelle Andeme Edzang 1 , Gabriel Canard 1 , Daniel Ferry 1 , Olivier Grauby 1 , Alain Ranguis 1 , Olivier Siri 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-10-11 , DOI: 10.1002/chem.201602288 Zhongrui Chen 1 , Rose Haddoub 1 , Jérôme Mahé 2 , Gabriel Marchand 2 , Denis Jacquemin 2, 3 , Judicaelle Andeme Edzang 1 , Gabriel Canard 1 , Daniel Ferry 1 , Olivier Grauby 1 , Alain Ranguis 1 , Olivier Siri 1
Affiliation
|
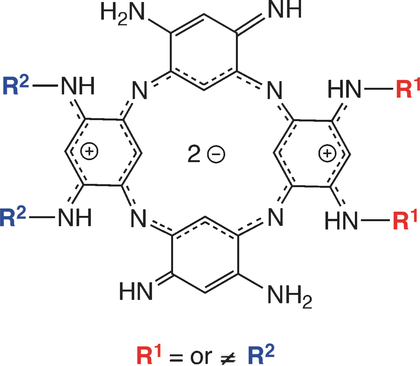
Pre‐ and postintroduction of substituents with respect to the macrocyclization step leads to previously unknown N‐substituted azacalixphyrins. The stepwise synthetic approach has been studied in detail to highlight the key role of the N‐substituents of the precursors and/or intermediates in terms of reactivity. Based on a combined experimental and theoretical investigation, the relationship between the properties of the macrocycles and their degree of substitution is rationalized. Depending on the nature of the N‐substituents, the formation of supramolecular ribbon‐like structures could also be observed, as demonstrated by combined TEM, SEM, AFM, and FTIR experiments.
中文翻译:

N取代的Azacalixphyrin:合成,性质和自组装
相对于大环化步骤而言,在引入取代基之前和之后会导致以前未知的N-取代的氮杂杯杂卟啉。已对逐步合成方法进行了详细研究,以突出前体和/或中间体的N取代基在反应性方面的关键作用。在实验和理论研究相结合的基础上,大环化合物的性质与其取代程度之间的关系得到合理化。根据N取代基的性质,也可以观察到超分子带状结构的形成,这通过TEM,SEM,AFM和FTIR组合实验得以证明。
更新日期:2016-10-11
中文翻译:

N取代的Azacalixphyrin:合成,性质和自组装
相对于大环化步骤而言,在引入取代基之前和之后会导致以前未知的N-取代的氮杂杯杂卟啉。已对逐步合成方法进行了详细研究,以突出前体和/或中间体的N取代基在反应性方面的关键作用。在实验和理论研究相结合的基础上,大环化合物的性质与其取代程度之间的关系得到合理化。根据N取代基的性质,也可以观察到超分子带状结构的形成,这通过TEM,SEM,AFM和FTIR组合实验得以证明。







































 京公网安备 11010802027423号
京公网安备 11010802027423号