当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring antiproliferative activities and kinase profile of ortho-substituted N-(4-(2-(benzylamino)-2-oxoethyl)phenyl)benzamides
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2023-10-24 , DOI: 10.1111/cbdd.14379 Yosra A Muhammad 1, 2 , Abdelsattar M Omar 1, 2, 3 , Farid Ahmed 4, 5 , Maan T Khayat 1 , Azizah M Malebari 1 , Sara M Ibrahim 6 , Shaza A Mass 1, 2 , Mahmoud A Elfaky 7 , Moustafa E El-Araby 1, 2
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2023-10-24 , DOI: 10.1111/cbdd.14379 Yosra A Muhammad 1, 2 , Abdelsattar M Omar 1, 2, 3 , Farid Ahmed 4, 5 , Maan T Khayat 1 , Azizah M Malebari 1 , Sara M Ibrahim 6 , Shaza A Mass 1, 2 , Mahmoud A Elfaky 7 , Moustafa E El-Araby 1, 2
Affiliation
|
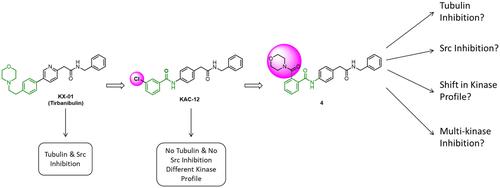
Designing kinase inhibitors that bind to the substrate site of oncogenic kinases in a promising, albeit less explored, approach to kinase inhibition as it was sought to avoid the issue of untoward off-target modulations. Our previously identified compound KAC-12 with a meta-chlorophenyl substitution was an example of this approach. While it showed confirmed inhibitory activity against cancer cells, this substitution shifted the profile of affected targets away from Src/tubulin which were seen with the parent KX-01. In this paper, we synthesized compounds with ortho-substitutions, and we investigated the effect of such substitutions on their cellular and subcellular activities. The compound N-(4-(2-(benzylamino)-2-oxoethyl)phenyl)-2-(morpholine-4-carbonyl)benzamide (4) exhibited substantial activities against cell lines such HCT116 (IC50 of 0.97 μM) and IC50 HL60 (2.84 μM). Kinase profiling showed that compound 4 trended consistently with KAC-12 as it did not affect Src, but it had more impact on members of the Src family of kinases (SFK) such as Yes, Hck, Fyn, Lck, and Lyn. Both compounds exhibited profound downregulation effects on Erk1/2 but differed on others such as GSK3α/β and C-Jun. Collectively, this study further support to the hypothesis that small structural changes might bring higher changes in their kinome profile.
中文翻译:

探索邻位取代的 N-(4-(2-(苄氨基)-2-氧代乙基)苯基)苯甲酰胺的抗增殖活性和激酶谱
设计与致癌激酶的底物位点结合的激酶抑制剂,这是一种很有前途但探索较少的激酶抑制方法,因为它试图避免不利的脱靶调节问题。我们之前鉴定的具有间氯苯基取代的化合物 KAC-12 就是这种方法的一个例子。虽然它显示出已证实的对癌细胞的抑制活性,但这种替代使受影响的靶标的特征远离了 Src/微管蛋白,这在母体 KX-01 中观察到。在本文中,我们合成了具有邻位取代的化合物,并研究了此类取代对其细胞和亚细胞活性的影响。化合物N -(4-(2-(苄氨基)-2-氧代乙基)苯基)-2-(吗啉-4-羰基)苯甲酰胺 ( 4 ) 对 HCT116 等细胞系表现出显着的活性(IC 50为 0.97 μM)和IC 50 HL60 (2.84 μM)。激酶分析显示,化合物4 的趋势与 KAC-12 一致,因为它不影响 Src,但它对 Src 激酶家族 (SFK) 的成员(例如 Yes、Hck、Fyn、Lck 和 Lyn)影响更大。两种化合物均对 Erk1/2 表现出深刻的下调作用,但对 GSK3α/β 和 C-Jun 等其他化合物的影响不同。总的来说,这项研究进一步支持了这样的假设:微小的结构变化可能会导致其激酶组谱发生更大的变化。
更新日期:2023-10-24
中文翻译:

探索邻位取代的 N-(4-(2-(苄氨基)-2-氧代乙基)苯基)苯甲酰胺的抗增殖活性和激酶谱
设计与致癌激酶的底物位点结合的激酶抑制剂,这是一种很有前途但探索较少的激酶抑制方法,因为它试图避免不利的脱靶调节问题。我们之前鉴定的具有间氯苯基取代的化合物 KAC-12 就是这种方法的一个例子。虽然它显示出已证实的对癌细胞的抑制活性,但这种替代使受影响的靶标的特征远离了 Src/微管蛋白,这在母体 KX-01 中观察到。在本文中,我们合成了具有邻位取代的化合物,并研究了此类取代对其细胞和亚细胞活性的影响。化合物N -(4-(2-(苄氨基)-2-氧代乙基)苯基)-2-(吗啉-4-羰基)苯甲酰胺 ( 4 ) 对 HCT116 等细胞系表现出显着的活性(IC 50为 0.97 μM)和IC 50 HL60 (2.84 μM)。激酶分析显示,化合物4 的趋势与 KAC-12 一致,因为它不影响 Src,但它对 Src 激酶家族 (SFK) 的成员(例如 Yes、Hck、Fyn、Lck 和 Lyn)影响更大。两种化合物均对 Erk1/2 表现出深刻的下调作用,但对 GSK3α/β 和 C-Jun 等其他化合物的影响不同。总的来说,这项研究进一步支持了这样的假设:微小的结构变化可能会导致其激酶组谱发生更大的变化。































 京公网安备 11010802027423号
京公网安备 11010802027423号