当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Perspectives of High-Performance Li–S Battery Electrolytes
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-10-22 , DOI: 10.1002/adfm.202309625 Jing Liu 1 , Yuhao Zhou 1 , Tianying Yan 1 , Xue‐Ping Gao 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-10-22 , DOI: 10.1002/adfm.202309625 Jing Liu 1 , Yuhao Zhou 1 , Tianying Yan 1 , Xue‐Ping Gao 1
Affiliation
|
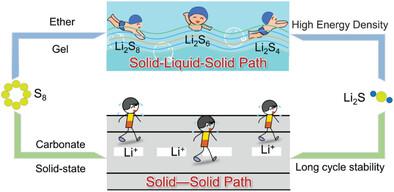
Lithium–sulfur batteries with high energy density are considered to be one of the most promising candidates for the next-generation energy storage devices. Electrolyte as the medium for Li+ transportation between the electrodes, also plays a crucial role in inhibiting the dissolution and diffusion of lithium polysulfides in Li–S batteries. The working mechanism of Li–S batteries in different electrolytes is classified into “solid-liquid-solid” and “solid-solid” conversions. Under the “solid-liquid-solid” conversion, Li–S batteries would inevitably face the challenges such as “shuttle effect” that lead to poor cycle performance, and under the “solid-solid” conversion, they would face interface mismatch that limits the utilization of sulfur with low energy density, while both conversion mechanisms cause uncontrollable Li dendrites on anode. According to the conversion mechanism, electrolytes can be divided into ether-based, ionic liquid-based, gel polymer electrolytes, and polymer-based solid-state electrolytes with “solid-liquid-solid” conversion, as well as carbonate-based electrolytes and oxide/sulfide-based solid-state electrolytes with “solid-solid” conversion. Based on the conversion mechanism of active materials in different electrolytes, the current status on the strategies from multiple perspectives are summarized to improve the electrochemical performance, with the hope to provide a comprehensive guideline toward the development of suitable electrolytes for Li–S batteries.
中文翻译:

高性能锂硫电池电解质的前景
具有高能量密度的锂硫电池被认为是下一代储能设备最有前途的候选者之一。电解质作为Li +在电极之间传输的介质,在抑制Li-S电池中多硫化锂的溶解和扩散方面也起着至关重要的作用。锂硫电池在不同电解质中的工作机理分为“固-液-固”和“固-固”转换。在“固-液-固”转换下,Li-S电池不可避免地面临“穿梭效应”等导致循环性能不佳的挑战,而在“固-固”转换下,又会面临界面失配的限制。利用低能量密度的硫,而两种转化机制都会导致负极上出现不可控的锂枝晶。根据转化机理,电解质可分为醚类、离子液体类、凝胶聚合物电解质、“固-液-固”转换的聚合物类固态电解质,以及碳酸酯类电解质和电解质类电解质。具有“固-固”转换的氧化物/硫化物基固态电解质。基于活性物质在不同电解质中的转化机理,从多个角度总结了提高电化学性能的策略现状,以期为开发适合Li-S电池的电解质提供全面的指导。
更新日期:2023-10-22
中文翻译:

高性能锂硫电池电解质的前景
具有高能量密度的锂硫电池被认为是下一代储能设备最有前途的候选者之一。电解质作为Li +在电极之间传输的介质,在抑制Li-S电池中多硫化锂的溶解和扩散方面也起着至关重要的作用。锂硫电池在不同电解质中的工作机理分为“固-液-固”和“固-固”转换。在“固-液-固”转换下,Li-S电池不可避免地面临“穿梭效应”等导致循环性能不佳的挑战,而在“固-固”转换下,又会面临界面失配的限制。利用低能量密度的硫,而两种转化机制都会导致负极上出现不可控的锂枝晶。根据转化机理,电解质可分为醚类、离子液体类、凝胶聚合物电解质、“固-液-固”转换的聚合物类固态电解质,以及碳酸酯类电解质和电解质类电解质。具有“固-固”转换的氧化物/硫化物基固态电解质。基于活性物质在不同电解质中的转化机理,从多个角度总结了提高电化学性能的策略现状,以期为开发适合Li-S电池的电解质提供全面的指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号