Molecular Therapy - Nucleic Acids ( IF 6.5 ) Pub Date : 2023-10-20 , DOI: 10.1016/j.omtn.2023.102063
Yiming Cheng 1 , Xiaochen Wang 1 , Shuyu Huang 1 , Liang Zhang 2 , Bei Lan 1 , Xuanyuan Li 1 , Hao Chen 1 , Zhenfeng Liu 1 , Yijie Su 1 , Lishan Xi 1 , Shengyun Feng 1 , Yanxuan Guo 1 , Jun Zhou 3 , Yingmei Wang 4 , Chenghao Xuan 1
|
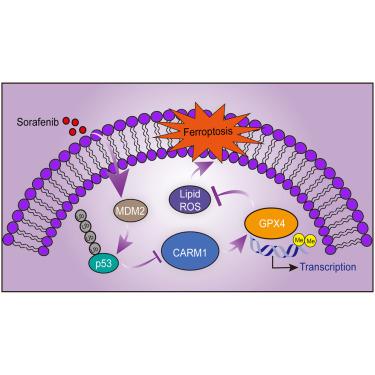
Ferroptosis is an iron-catalyzed form of regulated cell death that results from the accumulation of lipid peroxidation products and reactive oxygen species to a lethal content. However, the transcriptional regulation of ferroptosis is not well understood. Sorafenib, a standard drug for hepatocellular carcinoma (HCC), induces ferroptosis in HCC cells. In this study, we conducted a CRISPR-Cas9 library screening targeting epigenetic factors and identified coactivator-associated arginine methyltransferase 1 (CARM1) as a critical inhibitor of ferroptosis. CARM1 depletion intensified Sorafenib-induced ferroptosis, resulting in decreased cell viability, reduced cellular glutathione level, increased lipid peroxidation, and altered mitochondrial crista structure. Additionally, we investigated a CARM1 inhibitor (CARM1i) as a potential ferroptosis inducer. Combining the CARM1i with Sorafenib enhanced the induction of ferroptosis. Notably, both CARM1 knockdown and CARM1i showed cooperative effects with Sorafenib in inhibiting HCC growth in mice. The underlying mechanism involves CARM1-catalyzed H3R26me2a on the promoter of glutathione peroxidase 4, leading to its transcriptional activation and subsequent ferroptosis inhibition. Furthermore, Sorafenib treatment induced the transcription of CARM1 through the MDM2-p53 axis. In summary, our findings establish CARM1 as a critical ferroptosis inhibitor and highlight the potential of CARM1is as novel ferroptosis inducers, providing promising therapeutic strategies for HCC treatment.
中文翻译:

CRISPR-Cas9 文库筛选发现 CARM1 是肝细胞癌细胞铁死亡的关键抑制剂
铁死亡是一种铁催化的受调节细胞死亡形式,是由于脂质过氧化产物和活性氧累积至致死浓度而导致的。然而,铁死亡的转录调控尚不清楚。索拉非尼是治疗肝细胞癌 (HCC) 的标准药物,可诱导 HCC 细胞铁死亡。在这项研究中,我们针对表观遗传因子进行了 CRISPR-Cas9 文库筛选,并鉴定了共激活剂相关精氨酸甲基转移酶 1 (CARM1) 作为铁死亡的关键抑制剂。CARM1耗竭加剧了索拉非尼诱导的铁死亡,导致细胞活力下降、细胞谷胱甘肽水平降低、脂质过氧化增加以及线粒体嵴结构改变。此外,我们研究了 CARM1 抑制剂 (CARM1i) 作为潜在的铁死亡诱导剂。CARM1i 与索拉非尼的结合增强了铁死亡的诱导。值得注意的是,CARM1敲低和 CARM1i 均显示出与索拉非尼在抑制小鼠 HCC 生长方面的协同作用。潜在机制涉及谷胱甘肽过氧化物酶 4 启动子上的 CARM1 催化 H3R26me2a,导致其转录激活和随后的铁死亡抑制。此外,索拉非尼治疗通过 MDM2-p53 轴诱导 CARM1 转录。总之,我们的研究结果确立了 CARM1 作为一种关键的铁死亡抑制剂,并强调了 CARM1 作为新型铁死亡诱导剂的潜力,为 HCC 治疗提供了有前途的治疗策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号