Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2023-10-21 , DOI: 10.1016/j.reactfunctpolym.2023.105757 Kiomars Zargoosh , Fatemeh Khalili , Hossein Moradi Aliabadi
|
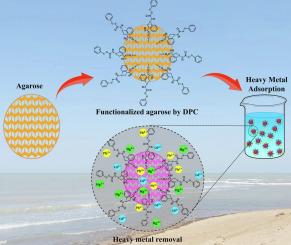
The real applicability of an adsorbent for the removal of heavy metal ions from large volumes of polluted waters critically depends on its cost, capacity, selectivity, and level of environmentally friendly nature of it. In this regard, an epichlorohydrin-activated agarose adsorbent was synthesized and functionalized by 1,5-diphenylcarbazone for the selective removal of Hg2+, Cd2+, and Pb2+ ions from aqueous solutions. The physicochemical properties of the adsorbent were characterized using FT-IR, FE-SEM, EDS, and BET analysis. The total pore volume and the specific surface area of the synthesized adsorbent were measured to be 0.023 m3.g−1 and 39 m2.g−1, respectively. The experimental maximum absorption capacities for Hg2+, Cd2+, and Pb2+ were obtained to be 307, 311, and 305 mg.g−1, respectively. In addition, the color of the adsorbent is changed during the adsorption of ions, thus this adsorbent can act as dual-function material for both the detection and adsorption of heavy metal ions. The adsorption isotherm and kinetics were well-fitted with the linear Freundlich and the linear pseudo-second-order models, respectively. The synthesized adsorbent was able to selectively adsorb target ions in the presence of possible interfering ions such as Fe3+, Fe2+, Al3+, Ca2+, K+ and Na+.
中文翻译:

1,5-二苯基卡巴腙官能化环氧活化琼脂糖的合成及其作为Hg2+、Cd2+和Pb2+离子高效吸附剂的应用
吸附剂从大量污染水中去除重金属离子的真正适用性关键取决于其成本、容量、选择性和环境友好性水平。在这方面,合成了环氧氯丙烷活化的琼脂糖吸附剂,并通过1,5-二苯基卡巴腙进行功能化,用于选择性去除水溶液中的Hg 2+、Cd 2+和Pb 2+离子。使用 FT-IR、FE-SEM、EDS 和 BET 分析对吸附剂的物理化学性质进行了表征。测得合成的吸附剂的总孔体积和比表面积分别为0.023m 3 .g -1和39m 2 .g -1 。获得的Hg 2+、Cd 2+和Pb 2+的实验最大吸收容量分别为307、311和305mg.g -1。此外,吸附剂在吸附离子过程中颜色会发生变化,因此该吸附剂可以作为重金属离子检测和吸附的双功能材料。吸附等温线和动力学分别与线性Freundlich 和线性伪二阶模型很好地拟合。合成的吸附剂能够在存在可能的干扰离子(例如Fe 3+、Fe 2+、Al 3+、Ca 2+、K +和Na + )的情况下选择性吸附目标离子。






























 京公网安备 11010802027423号
京公网安备 11010802027423号