Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-10-21 , DOI: 10.1016/j.bmc.2023.117507 Po-Chang Shih, Miyako Naganuma, Genichiro Tsuji, Yosuke Demizu, Mikihiko Naito
|
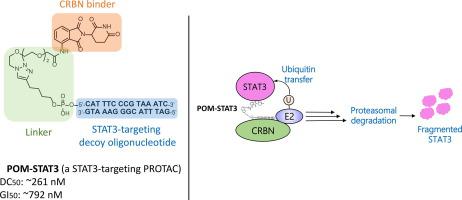
Proteolysis-targeting chimera (PROTAC) technology is a disruptive innovation in the drug development community, and over 20 PROTAC molecules are currently under clinical evaluation. These PROTAC molecules contain small-molecule warheads that bind to target proteins. Recently, oligonucleotide-warheaded PROTACs have emerged as a promising new tool to degrade DNA-binding proteins such as transcription factors. In this study, we applied an oligonucleotide-warheaded PROTAC technology to induce the degradation of signal transducer and activator of transcription 3 (STAT3), which is a hard-to-target protein. A double-stranded decoy oligonucleotide specific to STAT3 was conjugated to E3 binders (pomalidomide, VH032, and LCL161) to generate PROTAC molecules that recruited different E3 ubiquitin ligases cereblon (CRBN), von Hippel-Lindau (VHL), and inhibitor of apoptosis protein (IAP), respectively. One of the resulting PROTAC molecules, POM-STAT3, which recruits CRBN, potently induces STAT3 degradation. STAT3 degradation by POM-STAT3 was abolished by scrambling the oligonucleotide sequences of POM-STAT3 and by adding a double-stranded decoy oligonucleotide against STAT3 in a competitive manner, suggesting the significance of oligonucleotide sequences in STAT3 degradation. Moreover, POM-STAT3-induced STAT3 degradation was suppressed by the CRBN binder thalidomide, proteasome inhibitor bortezomib, E1 inhibitor MLN7243, and siRNA-mediated depletion of CRBN, indicating that STAT3 degradation is mediated by the ubiquitin-proteasome system, which involves CRBN as the responsible E3 ubiquitin ligase. Consistent with STAT3 degradation, NCI-H2087 cell viability was severely reduced following POM-STAT3 treatment. Thus, POM-STAT3 is a STAT3 degrader that potentially has cytocidal activity against cancer cells that are highly dependent on STAT3 signaling, which implies that inducing protein degradation by decoy oligonucleotide-warheaded PROTAC molecules could be harnessed to be therapeutic against oncogenic transcription factors.
中文翻译:

开发针对 STAT3 的诱饵寡核苷酸弹头嵌合分子
蛋白水解靶向嵌合体(PROTAC)技术是药物开发领域的颠覆性创新,目前有超过20种PROTAC分子正在接受临床评估。这些 PROTAC 分子含有与目标蛋白质结合的小分子弹头。最近,寡核苷酸弹头 PROTAC 已成为一种有前景的新工具,可降解 DNA 结合蛋白(如转录因子)。在本研究中,我们应用寡核苷酸弹头PROTAC技术来诱导信号转导子和转录激活子3(STAT3)的降解,这是一种难以靶向的蛋白质。将 STAT3 特异性的双链诱饵寡核苷酸与 E3 结合剂(泊马度胺、VH032 和 LCL161)缀合,生成招募不同 E3 泛素连接酶 cereblon (CRBN)、von Hippel-Lindau (VHL) 和凋亡蛋白抑制剂的 PROTAC 分子(IAP),分别。由此产生的 PROTAC 分子之一 POM-STAT3 可招募 CRBN,有效诱导 STAT3 降解。通过打乱POM-STAT3的寡核苷酸序列并以竞争性方式添加针对STAT3的双链诱饵寡核苷酸,消除了POM-STAT3对STAT3的降解,这表明寡核苷酸序列在STAT3降解中的重要性。此外,POM-STAT3诱导的STAT3降解被CRBN结合剂沙利度胺、蛋白酶体抑制剂硼替佐米、E1抑制剂MLN7243和siRNA介导的CRBN消耗所抑制,表明STAT3降解是由泛素蛋白酶体系统介导的,其中CRBN作为负责的 E3 泛素连接酶。与 STAT3 降解一致,POM-STAT3 处理后 NCI-H2087 细胞活力严重降低。因此,POM-STAT3是一种STAT3降解剂,对高度依赖STAT3信号传导的癌细胞具有潜在的杀细胞活性,这意味着通过诱饵寡核苷酸弹头PROTAC分子诱导蛋白质降解可用于治疗致癌转录因子。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号