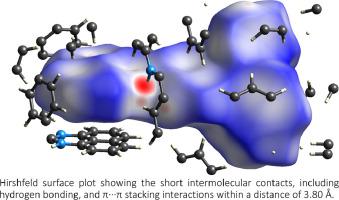
近萘并稠合嘧啶衍生物,称为 1H-perimidines 或 1H-苯并[ d,e ]喹唑啉,最近已被研究用于多种应用。通过 1,8-二氨基萘与适当的醛的缩合反应合成了一系列 2-取代-1H-perimidine 化合物 (1A–1F)。2-取代-1H-哌啶鎓碘化物盐(1A + I – –1F + I – ) 是通过在弱碱K 2 CO 3存在下使用甲基碘将1H-哌啶鎓甲基化获得的。使用NaBH 4在甲醇中还原perimidine 盐,得到2-取代-2,3-二氢-1H-perimidine 化合物(1AH–1FH)。通过1 H、13 C-NMR 和 UV-Vis 吸收光谱、元素分析对化合物进行了表征,对于化合物 1C、1B + I –、1C + I –、1E + I –、1F + I –,和 1BH–1EH,它们的分子结构已使用单晶 X 射线分析确定,并与相关 1H-perimidine 衍生物种的分子结构进行比较。比较了1C、1C + I –和 1CH固态结构的晶体堆积,揭示了分子 IC 通过分子间 N–H·N 氢键和 π·π 堆积相互作用进一步稳定性,同时在分子 1C + I –和 1CH的情况下,通过两个相邻的二甲酰亚胺环之间的短原子间 C–H…C 接触。为了进一步量化分子间相互作用并研究它们对每个分子中晶体堆积的贡献,进行了赫什菲尔德表面分析。使用循环伏安法研究了periminium盐1A + I – –1F + I –和氢化物还原物质1AH–1FH的氧化还原性质,并与N-DMBI-H ((4-(1,3-二甲基-2,3-二氢-1H-苯并[ d ]咪唑-2-基)苯基)二甲胺)。盐类表现出不可逆的还原波,如 2-取代-1,3-二甲基-1H-苯并[d]咪唑鎓盐,而氢化物则经历可逆的氧化过程,峰值比 ( I pc / I pa ) > 0.5。这显示了 1H •+物质在 100 mV s –1扫描速率下的化学稳定性。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Synthesis, X-ray structures, Hirshfeld Surface Analysis, and Redox Behaviour of 2-substituted-1H-perimidine derivatives
Peri-naptho fused pyrimidine derivatives, known as 1H-perimidines or 1H-benzo[d,e]quinazolines, have recently been studied for a diverse range of applications. A series of 2-substituted-1H-perimidine compounds (1A–1F) have been synthesized by the condensation reaction of 1,8-diaminonapthlene with the appropriate aldehyde. The 2-substituted-1H-perimidinium iodide salts (1A+I––1F+I–) were obtained by methylating 1H-perimidine using methyl iodide in the presence of a mild base K2CO3. The perimidinium salts were reduced using NaBH4 in methanol to obtain 2-substituted-2,3-dihydro-1H-perimidine compounds (1AH–1FH). The compounds were characterized by 1H, 13C-NMR and UV-Vis absorption spectroscopy, elemental analysis, and in the case of compounds 1C, 1B+I–, 1C+I–, 1E+I–, 1F+I–, and 1BH–1EH, their molecular structures have been determined using single crystal X-ray analysis and compared to those of related 1H-perimidine derived species. The crystal packings from the solid-state structures of 1C, 1C+I–, and 1CH were compared and revealed further stability of the molecule IC through intermolecular N–H···N hydrogen bonding and π···π stacking interactions, while in the case of molecules 1C+I–, and 1CH through short interatomic C–H···C contacts between two adjacent perimidine rings. To further quantify the intermolecular interactions and investigate their contributions to the crystal packing in each molecule, Hirshfeld surface analysis was performed. The redox properties of the perimidinium salts 1A+I––1F+I–, and the hydride reduced species 1AH–1FH were studied using cyclic voltammetry, and compared to that of N-DMBI-H ((4-(1,3-dimethyl-2,3-dihydro-1H-benzo[d]imidazole-2-yl)phenyl)dimethylamine). The salts exhibit an irreversible reduction wave, like those of 2-substituted-1,3-dimethyl-1H-benzo[d]imidazolium salts, while the hydrides undergo a reversible oxidation process, with the peak ratio (Ipc/Ipa) > 0.5. This shows the chemical stability of the 1H•+ species at a scan rate of 100 mV s–1.


































 京公网安备 11010802027423号
京公网安备 11010802027423号