当前位置:
X-MOL 学术
›
Biomed. Pharmacother.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The 3-(3-oxoisoindolin-1-yl)pentane-2,4-dione (ISOAC1) as a new molecule able to inhibit Amyloid β aggregation and neurotoxicity
Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2023-10-21 , DOI: 10.1016/j.biopha.2023.115745 Ilaria Piccialli 1 , Francesca Greco 2 , Giovanni Roviello 3 , Maria Josè Sisalli 1 , Valentina Tedeschi 1 , Antonia di Mola 4 , Nicola Borbone 2 , Giorgia Oliviero 5 , Vincenzo De Feo 6 , Agnese Secondo 1 , Antonio Massa 4 , Anna Pannaccione 1
Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2023-10-21 , DOI: 10.1016/j.biopha.2023.115745 Ilaria Piccialli 1 , Francesca Greco 2 , Giovanni Roviello 3 , Maria Josè Sisalli 1 , Valentina Tedeschi 1 , Antonia di Mola 4 , Nicola Borbone 2 , Giorgia Oliviero 5 , Vincenzo De Feo 6 , Agnese Secondo 1 , Antonio Massa 4 , Anna Pannaccione 1
Affiliation
|
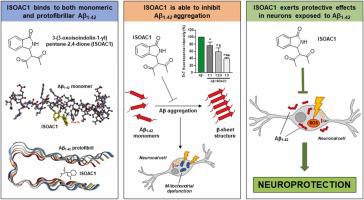
Amyloid β 1–42 (Aβ1–42 ) protein aggregation is considered one of the main triggers of Alzheimer’s disease (AD). In this study, we examined the in vitro anti-amyloidogenic activity of the isoindolinone derivative 3-(3-oxoisoindolin-1-yl)pentane-2,4-dione (ISOAC1) and its neuroprotective potential against the Aβ1–42 toxicity. By performing the Thioflavin T fluorescence assay, Western blotting analyses, and Circular Dichroism experiments, we found that ISOAC1 was able to reduce the Aβ1–42 aggregation and conformational transition towards β-sheet structures. Interestingly, in silico studies revealed that ISOAC1 was able to bind to both the monomer and a pentameric protofibril of Aβ1–42 , establishing a hydrophobic interaction with the PHE19 residue of the Aβ1–42 KLVFF motif. In vitro analyses on primary cortical neurons showed that ISOAC1 counteracted the increase of intracellular Ca2+ levels and decreased the Aβ1–42 -induced toxicity, in terms of mitochondrial activity reduction and increase of reactive oxygen species production. In addition, confocal microscopy analyses showed that ISOAC1 was able to reduce the Aβ1–42 intraneuronal accumulation. Collectively, our results clearly show that ISOAC1 exerts a neuroprotective effect by reducing the Aβ1–42 aggregation and toxicity, hence emerging as a promising compound for the development of new Aβ-targeting therapeutic strategies for AD treatment.
中文翻译:

3-(3-氧代异吲哚啉-1-基)戊烷-2,4-二酮 (ISOAC1) 作为一种能够抑制淀粉样蛋白β聚集和神经毒性的新分子
淀粉样蛋白 β 1-42 (Aβ1-42) 蛋白聚集被认为是阿尔茨海默病 (AD) 的主要触发因素之一。在这项研究中,我们检查了异吲哚酮衍生物 3-(3-氧代异吲哚啉-1-基)戊烷-2,4-二酮 (ISOAC1) 的体外抗淀粉样蛋白生成活性及其对 Aβ1-42 毒性的神经保护潜力。通过进行硫黄素 T 荧光测定、蛋白质印迹分析和圆二色谱实验,我们发现 ISOAC1 能够减少 Aβ1-42 聚集和向 β 折叠结构的构象转变。有趣的是,计算机研究表明,ISOAC1 能够与 Aβ1-42 的单体和五聚体原纤维结合,与 Aβ1-42 KLVFF 基序的 PHE19 残基建立疏水相互作用。对原代皮层神经元的体外分析表明,ISOAC1 在线粒体活性降低和活性氧产生增加方面抵消了细胞内 Ca2+ 水平的增加,并降低了 Aβ1-42 诱导的毒性。此外,共聚焦显微镜分析表明,ISOAC1 能够减少 Aβ1-42 神经元内积累。总的来说,我们的结果清楚地表明,ISOAC1 通过减少 Aβ1-42 聚集和毒性来发挥神经保护作用,因此成为开发新的 Aβ 靶向治疗策略的有前途的化合物。
更新日期:2023-10-21
中文翻译:

3-(3-氧代异吲哚啉-1-基)戊烷-2,4-二酮 (ISOAC1) 作为一种能够抑制淀粉样蛋白β聚集和神经毒性的新分子
淀粉样蛋白 β 1-42 (Aβ1-42) 蛋白聚集被认为是阿尔茨海默病 (AD) 的主要触发因素之一。在这项研究中,我们检查了异吲哚酮衍生物 3-(3-氧代异吲哚啉-1-基)戊烷-2,4-二酮 (ISOAC1) 的体外抗淀粉样蛋白生成活性及其对 Aβ1-42 毒性的神经保护潜力。通过进行硫黄素 T 荧光测定、蛋白质印迹分析和圆二色谱实验,我们发现 ISOAC1 能够减少 Aβ1-42 聚集和向 β 折叠结构的构象转变。有趣的是,计算机研究表明,ISOAC1 能够与 Aβ1-42 的单体和五聚体原纤维结合,与 Aβ1-42 KLVFF 基序的 PHE19 残基建立疏水相互作用。对原代皮层神经元的体外分析表明,ISOAC1 在线粒体活性降低和活性氧产生增加方面抵消了细胞内 Ca2+ 水平的增加,并降低了 Aβ1-42 诱导的毒性。此外,共聚焦显微镜分析表明,ISOAC1 能够减少 Aβ1-42 神经元内积累。总的来说,我们的结果清楚地表明,ISOAC1 通过减少 Aβ1-42 聚集和毒性来发挥神经保护作用,因此成为开发新的 Aβ 靶向治疗策略的有前途的化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号