当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Technetium Nitrido Complexes of Tetradentate Thiosemicarbazones: Kit-Based Radiolabeling, Characterization, and In Vivo Evaluation
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-10-19 , DOI: 10.1021/acs.inorgchem.3c02650
Cormac A A Kelderman 1 , Rachel C Maclean 1, 2 , Ingebjørg N Hungnes 3 , Patrick R W J Davey 1, 4 , Ekaterina Salimova 5 , Michael de Veer 5 , Natasha Patel 3 , Michelle T Ma 3 , Brett M Paterson 1, 2, 5
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-10-19 , DOI: 10.1021/acs.inorgchem.3c02650
Cormac A A Kelderman 1 , Rachel C Maclean 1, 2 , Ingebjørg N Hungnes 3 , Patrick R W J Davey 1, 4 , Ekaterina Salimova 5 , Michael de Veer 5 , Natasha Patel 3 , Michelle T Ma 3 , Brett M Paterson 1, 2, 5
Affiliation
|
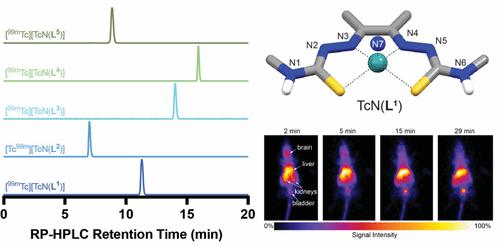
Bis(thiosemicarbazone) and pyridylhydrazone-thiosemicarbazone chelators have demonstrated utility in nuclear medicine. In particular, the 64Cu2+ complexes have been extensively developed for hypoxia imaging and molecular imaging of peptide and protein markers of disease. However, the chemistry and application of bis(thiosemicarbazone) and pyridylhydrazone-thiosemicarbazone chelators in combination with 99mTc, the most widely used radionuclide in nuclear medicine, is underexplored. Herein, a series of bis(thiosemicarbazone) and pyridylhydrazone-thiosemicarbazone chelators were radiolabeled with nitrido-technetium-99m in an optimized one-pot synthesis from [99mTc]TcO4–. Optimization of the radiochemical syntheses allowed for production of the complexes in >90% radiochemical conversion with apparent molar activities of 3.3–5 GBq/μmol. Competition experiments demonstrated the excellent stability of the complexes. The nitrido-technetium-99 complexes were synthesized, and the chemical identities were investigated using mass spectrometry, spectroscopy, and density functional theory calculations. Complexation of nitrido-rhenium(V) was achieved with the N4-dialkylated bis(thiosemicarbazones). Planar imaging and ex vivo biodistribution studies of the five 99mTc complexes were conducted on healthy BALB/c mice to determine in vivo behavior. The lipophilic nature of the complexes resulted in uptake of 1.6–5.7% ID g–1 in the brain at 2 min postinjection and retention of 0.4–1.7% ID g–1 at 15 min postinjection. The stability of the complexes and the biodistribution data demonstrate that these chelators are ideal platforms for future production of radiopharmaceutical candidates.
中文翻译:

四齿缩氨基硫脲的亚氮锝配合物:基于试剂盒的放射性标记、表征和体内评估
双(缩氨基硫脲)和吡啶基腙-缩氨基硫脲螯合剂已被证明在核医学中具有实用性。特别是, 64 Cu 2+复合物已被广泛开发用于缺氧成像以及疾病肽和蛋白质标记物的分子成像。然而,双(缩氨基硫脲)和吡啶腙-缩氨基硫脲螯合剂与核医学中最广泛使用的放射性核素99m Tc 结合的化学和应用尚未得到充分研究。在此,一系列双(缩氨基硫脲)和吡啶基腙-缩氨基硫脲螯合剂在[ 99m Tc]TcO 4 –的优化一锅合成中用硝基锝-99m 进行放射性标记。放射化学合成的优化允许以 >90% 的放射化学转化率生产配合物,表观摩尔活度为 3.3–5 GBq/μmol。竞争实验证明了该配合物具有优异的稳定性。合成了硝基-锝-99 配合物,并使用质谱、光谱和密度泛函理论计算研究了化学特性。硝基铼(V)与N 4 -二烷基化双(缩氨基硫脲)的络合得以实现。对健康 BALB/c 小鼠进行了五种99m Tc 复合物的平面成像和离体生物分布研究,以确定体内行为。复合物的亲脂性导致注射后 2 分钟大脑吸收 1.6–5.7% ID g –1 ,注射后 15 分钟保留 0.4–1.7% ID g –1 。 复合物的稳定性和生物分布数据表明这些螯合剂是未来生产放射性药物候选物的理想平台。
更新日期:2023-10-19
中文翻译:

四齿缩氨基硫脲的亚氮锝配合物:基于试剂盒的放射性标记、表征和体内评估
双(缩氨基硫脲)和吡啶基腙-缩氨基硫脲螯合剂已被证明在核医学中具有实用性。特别是, 64 Cu 2+复合物已被广泛开发用于缺氧成像以及疾病肽和蛋白质标记物的分子成像。然而,双(缩氨基硫脲)和吡啶腙-缩氨基硫脲螯合剂与核医学中最广泛使用的放射性核素99m Tc 结合的化学和应用尚未得到充分研究。在此,一系列双(缩氨基硫脲)和吡啶基腙-缩氨基硫脲螯合剂在[ 99m Tc]TcO 4 –的优化一锅合成中用硝基锝-99m 进行放射性标记。放射化学合成的优化允许以 >90% 的放射化学转化率生产配合物,表观摩尔活度为 3.3–5 GBq/μmol。竞争实验证明了该配合物具有优异的稳定性。合成了硝基-锝-99 配合物,并使用质谱、光谱和密度泛函理论计算研究了化学特性。硝基铼(V)与N 4 -二烷基化双(缩氨基硫脲)的络合得以实现。对健康 BALB/c 小鼠进行了五种99m Tc 复合物的平面成像和离体生物分布研究,以确定体内行为。复合物的亲脂性导致注射后 2 分钟大脑吸收 1.6–5.7% ID g –1 ,注射后 15 分钟保留 0.4–1.7% ID g –1 。 复合物的稳定性和生物分布数据表明这些螯合剂是未来生产放射性药物候选物的理想平台。

































 京公网安备 11010802027423号
京公网安备 11010802027423号