当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Synthesis of a Macrocyclic Peptide-Like C5aR Antagonist for Intravenous Applications
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-10-19 , DOI: 10.1021/acs.oprd.3c00202
Yiqing Feng 1 , Sidney Liang 1 , Jonathan Langille 1 , Betsy S. Pierce 1 , SeungWon Chung 1 , Jan Szeliga 1 , Glenn Wilcox 1 , Paul Simonds 1 , Kathleen A. Farley 1 , Bryan Li 1 , Carmen Garcia-Irizarry 1 , Peter Jones 1 , Ricardo Lira 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-10-19 , DOI: 10.1021/acs.oprd.3c00202
Yiqing Feng 1 , Sidney Liang 1 , Jonathan Langille 1 , Betsy S. Pierce 1 , SeungWon Chung 1 , Jan Szeliga 1 , Glenn Wilcox 1 , Paul Simonds 1 , Kathleen A. Farley 1 , Bryan Li 1 , Carmen Garcia-Irizarry 1 , Peter Jones 1 , Ricardo Lira 1
Affiliation
|
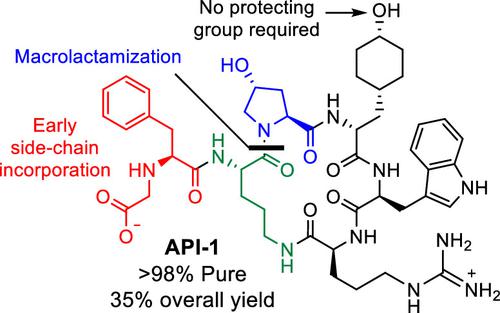
A multigram scale synthetic procedure for the preparation of a complex and polar macrocyclic peptidic C5aR antagonist is described. The route was developed through improvements to an initial small-scale research synthesis and hinged on optimized solid-phase peptide synthesis featuring an early side chain decoration, a highly efficient off-resin macrolactamization, and global deprotection steps. These improvements resulted in a reduction in off-resin peptide manipulations and ultimately to a 6-fold increase in overall yield from 2-chlorotrityl-bound intermediate SP-7c.
中文翻译:

用于静脉注射的大环肽样 C5aR 拮抗剂的改进合成
描述了用于制备复杂的极性大环肽 C5aR 拮抗剂的多克规模合成程序。该路线是通过对最初小规模研究合成的改进而开发的,并取决于优化的固相肽合成,其特点是早期侧链装饰、高效的树脂外大环内酰胺化和全局脱保护步骤。这些改进减少了树脂外肽操作,最终使 2-氯三苯甲基结合的中间体SP-7c 的总产率增加了 6 倍。
更新日期:2023-10-19
中文翻译:

用于静脉注射的大环肽样 C5aR 拮抗剂的改进合成
描述了用于制备复杂的极性大环肽 C5aR 拮抗剂的多克规模合成程序。该路线是通过对最初小规模研究合成的改进而开发的,并取决于优化的固相肽合成,其特点是早期侧链装饰、高效的树脂外大环内酰胺化和全局脱保护步骤。这些改进减少了树脂外肽操作,最终使 2-氯三苯甲基结合的中间体SP-7c 的总产率增加了 6 倍。

































 京公网安备 11010802027423号
京公网安备 11010802027423号