Synlett ( IF 1.7 ) Pub Date : 2023-10-19 , DOI: 10.1055/s-0042-1751507
Thazha P. Prakash 1 , Jinghua Yu 1 , Guillermo Vasquez 1 , Charels R. Allerson 2 , Eric E. Swayze 1
|
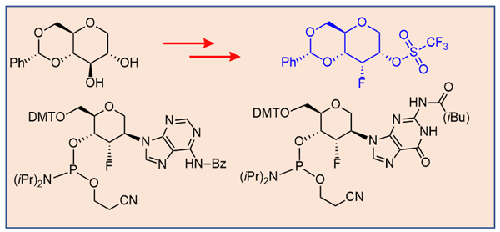
We report a convenient and scalable synthetic approach for the synthesis of 3′-fluoro hexitol adenosine and guanosine nucleoside analogues and corresponding phosphoramidites in good yield. 1,5-Anhydro-4,6-O-benzylidene-
中文翻译:

3′-氟己糖醇腺苷和鸟苷亚磷酰胺的简便合成
我们报告了一种方便且可扩展的合成方法,用于以良好的收率合成 3'-氟己糖醇腺苷和鸟苷核苷类似物以及相应的亚磷酰胺。1,5-脱水-4,6- O-亚苄基-

































 京公网安备 11010802027423号
京公网安备 11010802027423号