当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Salt and Cocrystals Combining Sulfathiazole with Pyrimethamine
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2023-10-20 , DOI: 10.1021/acs.cgd.3c00626
Laurie Bodart 1 , Jeanne Body 1 , Nikolay Tumanov 1 , Tom Leyssens 2 , Johan Wouters 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2023-10-20 , DOI: 10.1021/acs.cgd.3c00626
Laurie Bodart 1 , Jeanne Body 1 , Nikolay Tumanov 1 , Tom Leyssens 2 , Johan Wouters 1
Affiliation
|
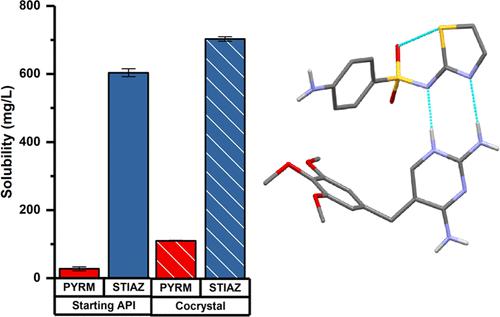
Dihydrofolate reductase inhibitors, such as pyrimethamine, are known to have synergistic effects with sulfonamides. Four new solid forms combining pyrimethamine and sulfathiazole, one cocrystal (pyrimethamine-sulfathiazole (1:1)) and three solvated salt cocrystals (in which pyrimethamine and sulfathiazole are in a 1:2 molar ratio), are determined and characterized. Desolvation of the salt cocrystals leads to either a physical mixture of the starting compounds or the formation of the cocrystal (1:1) with an excess of sulfathiazole. The pyrimethamine sulfathiazole binary phase diagram was determined, which reveals that the pyrimethamine-sulfathiazole (1:2) composition point corresponds to a metastable eutectic. Obtaining this metastable eutectic as a pure powder is therefore rather difficult since it converts to a stable mixture of the (1:1) cocrystal + sulfathiazole. The abovementioned drug–drug multicomponent systems are characterized in terms of thermal stability (by TGA/DSC) and solubility. Both pyrimethamine-sulfathiazole (1:1) cocrystal and pyrimethamine-sulfathiazole (1:2) ethanol-solvated salt cocrystal result in an increase of the parent drugs’ solubility.
中文翻译:

磺胺噻唑与乙胺嘧啶组合的盐和共晶体
已知二氢叶酸还原酶抑制剂,例如乙胺嘧啶,与磺胺类药物具有协同作用。测定并表征了乙胺嘧啶和磺胺噻唑的四种新固体形式,一种共晶体(乙胺嘧啶-磺胺噻唑(1:1))和三种溶剂化盐共晶体(其中乙胺嘧啶和磺胺噻唑的摩尔比为 1:2)。盐共晶的去溶剂化导致起始化合物的物理混合物或与过量磺胺噻唑形成共晶(1:1)。测定了乙胺嘧啶磺胺噻唑二元相图,表明乙胺嘧啶-磺胺噻唑(1:2)组成点对应于亚稳态共晶。因此,获得这种亚稳态共晶纯粉末相当困难,因为它会转化为 (1:1) 共晶 + 磺胺噻唑的稳定混合物。上述药物-药物多组分系统的特征在于热稳定性(通过 TGA/DSC)和溶解度。乙胺嘧啶-磺胺噻唑(1:1)共晶和乙胺嘧啶-磺胺噻唑(1:2)乙醇溶剂化盐共晶都会导致母药溶解度增加。
更新日期:2023-10-20
中文翻译:

磺胺噻唑与乙胺嘧啶组合的盐和共晶体
已知二氢叶酸还原酶抑制剂,例如乙胺嘧啶,与磺胺类药物具有协同作用。测定并表征了乙胺嘧啶和磺胺噻唑的四种新固体形式,一种共晶体(乙胺嘧啶-磺胺噻唑(1:1))和三种溶剂化盐共晶体(其中乙胺嘧啶和磺胺噻唑的摩尔比为 1:2)。盐共晶的去溶剂化导致起始化合物的物理混合物或与过量磺胺噻唑形成共晶(1:1)。测定了乙胺嘧啶磺胺噻唑二元相图,表明乙胺嘧啶-磺胺噻唑(1:2)组成点对应于亚稳态共晶。因此,获得这种亚稳态共晶纯粉末相当困难,因为它会转化为 (1:1) 共晶 + 磺胺噻唑的稳定混合物。上述药物-药物多组分系统的特征在于热稳定性(通过 TGA/DSC)和溶解度。乙胺嘧啶-磺胺噻唑(1:1)共晶和乙胺嘧啶-磺胺噻唑(1:2)乙醇溶剂化盐共晶都会导致母药溶解度增加。































 京公网安备 11010802027423号
京公网安备 11010802027423号