当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cations Mediate Lithium Polysulfide Adsorption in Metal–Organic Frameworks for Lithium–Sulfur Batteries
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-10-19 , DOI: 10.1021/acs.jpcc.3c05539
Roberto A. Jarrín 1 , Kevin Bennett 2 , V. Sara Thoi 2, 3 , Brandon C. Bukowski 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-10-19 , DOI: 10.1021/acs.jpcc.3c05539
Roberto A. Jarrín 1 , Kevin Bennett 2 , V. Sara Thoi 2, 3 , Brandon C. Bukowski 1
Affiliation
|
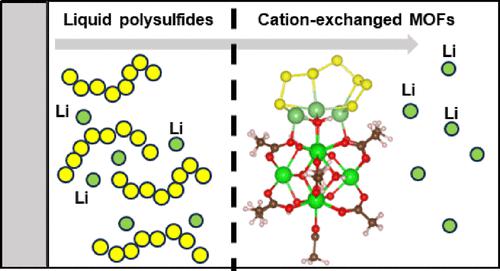
Lithium–sulfur (Li–S) batteries are one promising alternative to Li-ion batteries due to their higher theoretical specific capacity and energy density. However, several technical challenges such as polysulfide shuttling remain. As liquid polysulfide diffusion into the electrolyte causes a loss of capacity, different material classes have been explored to anchor lithium polysulfides and reduce the active material loss. The metal–organic framework (MOF) UiO-66 has been identified as one candidate material due to its porosity, high surface area, and zirconium oxide nodes that could anchor liquid polysulfides. MOFs also allow for postsynthetic modifications that can increase their adsorption specificity toward liquid polysulfides and reduce shuttling. In this work, we combined atomistic simulations and experimental characterization to probe the molecular interactions between lithium polysulfides and functionalized UiO-66 nodes. We explored how lithium polysulfides adsorb to open sites caused by missing linker defects as well as sites functionalized with alkali cations. Our results demonstrate that lithium polysulfides adsorb favorably to UiO-66 through Li–O electrostatic interactions. In addition, we found that nodes functionalized with alkali metals demonstrated stronger adsorption of long-chain lithium polysulfides (Li2S4–8) by facilitating charge transfer to the nodes. Experimental ultraviolet-visible and 7Li NMR measurements on Zr polyoxometalates and UiO-66 provided further evidence that lithiation favors adsorption of long-chain polysulfides. Our findings indicate that UiO-66 functionalization plays an important role in polysulfide adsorption, which may have implications in controlling the shuttle effect. The fundamental insights into polysulfide adsorption shown here provide quantitative principles to design functionalized moieties and further inhibit polysulfide shuttling.
中文翻译:

阳离子介导锂硫电池金属有机框架中多硫化锂的吸附
锂硫(Li-S)电池因其较高的理论比容量和能量密度而成为锂离子电池的一种有前途的替代品。然而,多硫化物穿梭等一些技术挑战仍然存在。由于液体多硫化物扩散到电解质中会导致容量损失,因此已经探索了不同的材料类别来固定多硫化锂并减少活性材料损失。金属有机骨架 (MOF) UiO-66 因其孔隙率、高表面积和可以锚定液体多硫化物的氧化锆节点而被确定为一种候选材料。MOF 还允许进行合成后修饰,从而提高其对液体多硫化物的吸附特异性并减少穿梭。在这项工作中,我们结合原子模拟和实验表征来探讨多硫化锂和功能化 UiO-66 节点之间的分子相互作用。我们探索了多硫化锂如何吸附到由缺失连接子缺陷引起的开放位点以及用碱金属阳离子功能化的位点。我们的结果表明,多硫化锂通过 Li-O 静电相互作用有利地吸附到 UiO-66。此外,我们发现用碱金属功能化的节点通过促进电荷转移到节点而表现出对长链多硫化锂(Li 2 S 4–8 )更强的吸附。Zr 多金属氧酸盐和 UiO-66 的实验紫外-可见光和7 Li NMR 测量提供了进一步的证据,证明锂化有利于长链多硫化物的吸附。我们的研究结果表明,UiO-66 官能化在多硫化物吸附中发挥着重要作用,这可能对控制穿梭效应有影响。这里展示的对多硫化物吸附的基本见解为设计功能化部分和进一步抑制多硫化物穿梭提供了定量原理。
更新日期:2023-10-19
中文翻译:

阳离子介导锂硫电池金属有机框架中多硫化锂的吸附
锂硫(Li-S)电池因其较高的理论比容量和能量密度而成为锂离子电池的一种有前途的替代品。然而,多硫化物穿梭等一些技术挑战仍然存在。由于液体多硫化物扩散到电解质中会导致容量损失,因此已经探索了不同的材料类别来固定多硫化锂并减少活性材料损失。金属有机骨架 (MOF) UiO-66 因其孔隙率、高表面积和可以锚定液体多硫化物的氧化锆节点而被确定为一种候选材料。MOF 还允许进行合成后修饰,从而提高其对液体多硫化物的吸附特异性并减少穿梭。在这项工作中,我们结合原子模拟和实验表征来探讨多硫化锂和功能化 UiO-66 节点之间的分子相互作用。我们探索了多硫化锂如何吸附到由缺失连接子缺陷引起的开放位点以及用碱金属阳离子功能化的位点。我们的结果表明,多硫化锂通过 Li-O 静电相互作用有利地吸附到 UiO-66。此外,我们发现用碱金属功能化的节点通过促进电荷转移到节点而表现出对长链多硫化锂(Li 2 S 4–8 )更强的吸附。Zr 多金属氧酸盐和 UiO-66 的实验紫外-可见光和7 Li NMR 测量提供了进一步的证据,证明锂化有利于长链多硫化物的吸附。我们的研究结果表明,UiO-66 官能化在多硫化物吸附中发挥着重要作用,这可能对控制穿梭效应有影响。这里展示的对多硫化物吸附的基本见解为设计功能化部分和进一步抑制多硫化物穿梭提供了定量原理。




































 京公网安备 11010802027423号
京公网安备 11010802027423号