当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PAR2 deficiency tunes inflammatory microenvironment to magnify STING signalling for mitigating cancer metastasis via anionic CRISPR/Cas9 nanoparticles
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2023-10-18 , DOI: 10.1016/j.jconrel.2023.10.017 Xiujuan Fu 1 , Jianbin Li 1 , Yue Wu 1 , Canquan Mao 1 , Yuhong Jiang 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2023-10-18 , DOI: 10.1016/j.jconrel.2023.10.017 Xiujuan Fu 1 , Jianbin Li 1 , Yue Wu 1 , Canquan Mao 1 , Yuhong Jiang 1
Affiliation
|
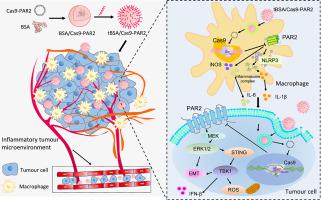
Metastasis is one of the most significant causes for deterioration of breast cancer, contributing to the clinical failure of anti-tumour drugs. Excessive inflammatory responses intensively promote the occurrence and development of tumour, while protease-activated receptor 2 (PAR2) as a cell membrane receptor actively participates in both tumour cell functions and inflammatory responses. However, rare investigations linked PAR2-mediated inflammatory environment to tumour progression. Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology is an emerging and powerful gene editing technique and can be applied for probing the new role of PAR2 in breast cancer metastasis, but it still needs the development of an efficient and safe delivery system. This work constructed anionic bovine serum albumin (BSA) nanoparticles to encapsulate CRISPR/Cas9 plasmid encoding PAR2 sgRNA and Cas9 (tBSA/Cas9-PAR2) for triggering PAR2 deficiency. tBSA/Cas9-PAR2 remarkably promoted CRISPR/Cas9 to enter and transfect both inflammatory and cancer cells, initiating precise PAR2 gene editing and . PAR2 deficiency by tBSA/Cas9-PAR2 effectively suppressed NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome signalling in inflammatory microenvironment to magnify stimulator of interferon genes (STING) signalling, reactive oxygen species (ROS) accumulation and epithelial-mesenchymal transition (EMT) reversal, consequently preventing breast cancer metastasis. Therefore, this study not only demonstrated the involvement and underlying mechanism of PAR2 in tumour progression modulating inflammatory microenvironment, but also suggested PAR2 deficiency by tBSA/Cas9-PAR2 as an attractive therapeutic strategy candidate for breast cancer metastasis.
中文翻译:

PAR2 缺陷调节炎症微环境,放大 STING 信号传导,通过阴离子 CRISPR/Cas9 纳米粒子减轻癌症转移
转移是乳腺癌恶化的最重要原因之一,导致抗肿瘤药物临床失败。过度的炎症反应强烈促进肿瘤的发生和发展,而蛋白酶激活受体2(PAR2)作为细胞膜受体积极参与肿瘤细胞功能和炎症反应。然而,很少有研究将 PAR2 介导的炎症环境与肿瘤进展联系起来。成簇规则间隔短回文重复序列(CRISPR)/Cas9技术是一种新兴而强大的基因编辑技术,可用于探索PAR2在乳腺癌转移中的新作用,但仍需要开发高效、安全的递送系统。这项工作构建了阴离子牛血清白蛋白 (BSA) 纳米粒子,以封装编码 PAR2 sgRNA 和 Cas9 (tBSA/Cas9-PAR2) 的 CRISPR/Cas9 质粒,以触发 PAR2 缺陷。 tBSA/Cas9-PAR2 显着促进 CRISPR/Cas9 进入并转染炎症细胞和癌细胞,启动精确的 PAR2 基因编辑和。 tBSA/Cas9-PAR2 引起的 PAR2 缺陷可有效抑制炎症微环境中的 NOD 样受体热蛋白结构域相关蛋白 3 (NLRP3) 炎症小体信号传导,从而放大干扰素基因 (STING) 信号传导刺激、活性氧 (ROS) 积累和上皮间质转变(EMT)逆转,从而防止乳腺癌转移。因此,这项研究不仅证明了 PAR2 在调节炎症微环境的肿瘤进展中的参与和潜在机制,而且还表明 tBSA/Cas9-PAR2 引起的 PAR2 缺陷是乳腺癌转移的一种有吸引力的候选治疗策略。
更新日期:2023-10-18
中文翻译:

PAR2 缺陷调节炎症微环境,放大 STING 信号传导,通过阴离子 CRISPR/Cas9 纳米粒子减轻癌症转移
转移是乳腺癌恶化的最重要原因之一,导致抗肿瘤药物临床失败。过度的炎症反应强烈促进肿瘤的发生和发展,而蛋白酶激活受体2(PAR2)作为细胞膜受体积极参与肿瘤细胞功能和炎症反应。然而,很少有研究将 PAR2 介导的炎症环境与肿瘤进展联系起来。成簇规则间隔短回文重复序列(CRISPR)/Cas9技术是一种新兴而强大的基因编辑技术,可用于探索PAR2在乳腺癌转移中的新作用,但仍需要开发高效、安全的递送系统。这项工作构建了阴离子牛血清白蛋白 (BSA) 纳米粒子,以封装编码 PAR2 sgRNA 和 Cas9 (tBSA/Cas9-PAR2) 的 CRISPR/Cas9 质粒,以触发 PAR2 缺陷。 tBSA/Cas9-PAR2 显着促进 CRISPR/Cas9 进入并转染炎症细胞和癌细胞,启动精确的 PAR2 基因编辑和。 tBSA/Cas9-PAR2 引起的 PAR2 缺陷可有效抑制炎症微环境中的 NOD 样受体热蛋白结构域相关蛋白 3 (NLRP3) 炎症小体信号传导,从而放大干扰素基因 (STING) 信号传导刺激、活性氧 (ROS) 积累和上皮间质转变(EMT)逆转,从而防止乳腺癌转移。因此,这项研究不仅证明了 PAR2 在调节炎症微环境的肿瘤进展中的参与和潜在机制,而且还表明 tBSA/Cas9-PAR2 引起的 PAR2 缺陷是乳腺癌转移的一种有吸引力的候选治疗策略。













































 京公网安备 11010802027423号
京公网安备 11010802027423号