Synthesis ( IF 2.2 ) Pub Date : 2023-10-18 , DOI: 10.1055/s-0042-1751636 Seetaram Mohapatra , Tapaswini Das , Sonali Priyadarshini Parida , Sabita Nayak
|
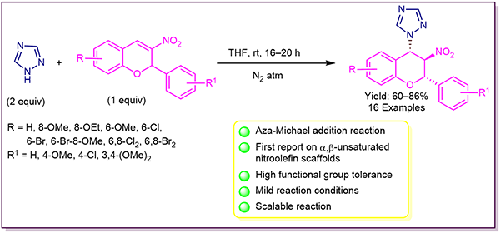
A simple and efficient aza-Michael addition reaction of 1,2,4-triazoles to functionalized 2-aryl-3-nitro-2H-chromenes has been demonstrated under catalyst- and base-free conditions. In this transformation, one intermolecular C–N bond formation is achieved at room temperature. A series of highly substituted 1,2,4-triazole-based 3-nitrochromanes were produced in good to excellent yields, up to 86%. The relative configuration of the Michael adducts was confirmed by X-ray crystallographic analysis. High yield, easy accessibility and a wide variety of functional group tolerance are the key features of this aza-Michael addition reaction.
中文翻译:

无催化剂、无碱条件下通过 Aza-Michael 加成反应合成高度取代的 1,2,4-三唑基 3-硝基色满
1,2,4-三唑与官能化2-芳基-3-硝基-2H-色烯的简单而有效的氮杂迈克尔加成反应已在无催化剂和无碱条件下得到证实。在这一转变中,在室温下形成一个分子间 C-N 键。一系列高度取代的 1,2,4-三唑基 3-硝基苯并二氢吡喃类化合物的产率从良好到优异,高达 86%。迈克尔加合物的相对构型通过X射线晶体学分析得到证实。高产率、易于获得和多种官能团耐受性是这种氮杂迈克尔加成反应的关键特征。































 京公网安备 11010802027423号
京公网安备 11010802027423号