当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isothiocyanates (in situ) and sulfonyl chlorides in water for N-functionalization of bicyclic amidines: access to N-alkylated γ-/ω-lactam derivatized thiourea and sulfonamides
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-10-19 , DOI: 10.1039/d3ob01584j Pankaj Kumar 1 , Aman Bhalla 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-10-19 , DOI: 10.1039/d3ob01584j Pankaj Kumar 1 , Aman Bhalla 1
Affiliation
|
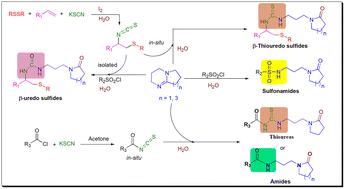
Herein, we showcase the potential of isothiocyanates generated in situ and aryl sulfonyl chlorides as electrophiles in water for N-functionalization of bicyclic amidines (DBN and DBU). This strategy provides complementary access to a range of thiouredosulfides, sulfonamides, aroylthioureas and amides derivativatized with distal γ- and ω-lactams. A novel sulfonyl chloride mediated formation of β-uredo sulfides has been achieved from β-isothiocyanato sulfides, removing the requirement for the harsh synthesis of unstable isocyanates. Mechanistic studies suggest a radical mechanism for the difunctionalization of alkenes, the efficacy of H2O in the ring opening of bicyclic amidines, and an oxygen source along with sulfonyl chloride as desulfurization agents for thiourea to afford urea derivatives.
中文翻译:

水中的异硫氰酸酯(原位)和磺酰氯用于双环脒的 N-官能化:获得 N-烷基化 γ-/ω-内酰胺衍生的硫脲和磺酰胺
在此,我们展示了原位生成的异硫氰酸酯和芳基磺酰氯作为水中亲电子试剂用于双环脒(DBN 和 DBU) N官能化的潜力。该策略提供了对一系列硫脲硫化物、磺酰胺、芳酰硫脲和用远端 γ-和 ω-内酰胺衍生的酰胺的补充途径。由β-异硫氰酸根硫化物实现了一种新型磺酰氯介导的β-乌脲硫化物的形成,消除了对不稳定异氰酸酯的苛刻合成的要求。机理研究提出了烯烃双官能化的根本机制、H 2 O在双环脒开环中的功效,以及氧源和磺酰氯作为硫脲脱硫剂以提供脲衍生物。
更新日期:2023-10-19
中文翻译:

水中的异硫氰酸酯(原位)和磺酰氯用于双环脒的 N-官能化:获得 N-烷基化 γ-/ω-内酰胺衍生的硫脲和磺酰胺
在此,我们展示了原位生成的异硫氰酸酯和芳基磺酰氯作为水中亲电子试剂用于双环脒(DBN 和 DBU) N官能化的潜力。该策略提供了对一系列硫脲硫化物、磺酰胺、芳酰硫脲和用远端 γ-和 ω-内酰胺衍生的酰胺的补充途径。由β-异硫氰酸根硫化物实现了一种新型磺酰氯介导的β-乌脲硫化物的形成,消除了对不稳定异氰酸酯的苛刻合成的要求。机理研究提出了烯烃双官能化的根本机制、H 2 O在双环脒开环中的功效,以及氧源和磺酰氯作为硫脲脱硫剂以提供脲衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号