当前位置:
X-MOL 学术
›
Nanoscale Adv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein engineering of multi-enzyme virus-like particle nanoreactors for enhanced chiral alcohol synthesis
Nanoscale Advances ( IF 4.6 ) Pub Date : 2023-10-18 , DOI: 10.1039/d3na00515a Taotao Feng 1 , Jiaxu Liu 1 , Xiaoyan Zhang 1 , Daidi Fan 2 , Yunpeng Bai 1
Nanoscale Advances ( IF 4.6 ) Pub Date : 2023-10-18 , DOI: 10.1039/d3na00515a Taotao Feng 1 , Jiaxu Liu 1 , Xiaoyan Zhang 1 , Daidi Fan 2 , Yunpeng Bai 1
Affiliation
|
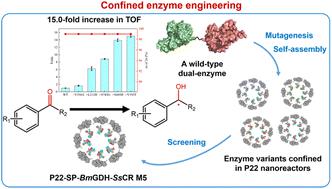
In the past decade, virus-like particles (VLPs) that can encapsulate single or multiple enzymes have been studied extensively as typical nanoreactors for biocatalysis in vitro, yet their catalytic efficiencies are usually inadequate for real applications. These biocatalytic nanoreactors should be engineered like their free-enzyme counterparts to improve their catalytic performance for potential applications. Herein we engineer biocatalytic VLPs for the enhanced synthesis of chiral alcohols. Different methods including directed evolution were applied to the entire bacteriophage P22 VLPs (except the coat protein), which encapsulated a carbonyl reductase from Scheffersomyces stipitis (SsCR) and a glucose dehydrogenase from Bacillus megaterium (BmGDH) in their capsids. The best variant, namely M5, showed an enhanced turnover frequency (TOF, min−1) up to 15-fold toward the majority of tested aromatic prochiral ketones, and gave up to 99% enantiomeric excess in the synthesis of chiral alcohol pharmaceutical intermediates. A comparison with the mutations of the free-enzyme counterparts showed that the same amino acid mutations led to different changes in the catalytic efficiencies of free and confined enzymes. Finally, the engineered M5 nanoreactor showed improved efficiency in the scale-up synthesis of chiral alcohols. The conversions of three substrates catalyzed by M5 were all higher than those catalyzed by the wild-type nanoreactor, demonstrating that enzyme-encapsulating VLPs can evolve to enhance their catalytic performance for potential applications.
中文翻译:

用于增强手性醇合成的多酶病毒样颗粒纳米反应器的蛋白质工程
在过去的十年中,可以封装单个或多个酶的病毒样颗粒(VLP)作为典型的体外生物催化纳米反应器已被广泛研究,但其催化效率通常不足以满足实际应用。这些生物催化纳米反应器应该像其游离酶对应物一样进行设计,以提高其潜在应用的催化性能。在此,我们设计生物催化 VLP 以增强手性醇的合成。对整个噬菌体 P22 VLP(外壳蛋白除外)应用了包括定向进化在内的不同方法,该噬菌体 P22 VLP 在其衣壳中封装了来自树干树干酵母( Ss CR) 的羰基还原酶 (Ss CR) 和来自巨大芽孢杆菌( Bm GDH) 的葡萄糖脱氢酶。最好的变体,即M5,对大多数测试的芳香族前手性酮显示出高达15倍的转换频率(TOF,min -1),并且在手性醇药物中间体的合成中给出高达99%的对映体过量。与游离酶对应物的突变比较表明,相同的氨基酸突变导致游离酶和限制酶的催化效率发生不同的变化。最后,工程化的 M5 纳米反应器在手性醇的放大合成中表现出更高的效率。M5 催化的三种底物的转化率均高于野生型纳米反应器催化的转化率,这表明酶封装的 VLP 可以进化以增强其潜在应用的催化性能。
更新日期:2023-10-18
中文翻译:

用于增强手性醇合成的多酶病毒样颗粒纳米反应器的蛋白质工程
在过去的十年中,可以封装单个或多个酶的病毒样颗粒(VLP)作为典型的体外生物催化纳米反应器已被广泛研究,但其催化效率通常不足以满足实际应用。这些生物催化纳米反应器应该像其游离酶对应物一样进行设计,以提高其潜在应用的催化性能。在此,我们设计生物催化 VLP 以增强手性醇的合成。对整个噬菌体 P22 VLP(外壳蛋白除外)应用了包括定向进化在内的不同方法,该噬菌体 P22 VLP 在其衣壳中封装了来自树干树干酵母( Ss CR) 的羰基还原酶 (Ss CR) 和来自巨大芽孢杆菌( Bm GDH) 的葡萄糖脱氢酶。最好的变体,即M5,对大多数测试的芳香族前手性酮显示出高达15倍的转换频率(TOF,min -1),并且在手性醇药物中间体的合成中给出高达99%的对映体过量。与游离酶对应物的突变比较表明,相同的氨基酸突变导致游离酶和限制酶的催化效率发生不同的变化。最后,工程化的 M5 纳米反应器在手性醇的放大合成中表现出更高的效率。M5 催化的三种底物的转化率均高于野生型纳米反应器催化的转化率,这表明酶封装的 VLP 可以进化以增强其潜在应用的催化性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号