International Journal of Pharmaceutics ( IF 5.3 ) Pub Date : 2023-10-17 , DOI: 10.1016/j.ijpharm.2023.123529 Yasmein Yaser Salem 1 , Gjylije Hoti 2 , Rana M F Sammour 1 , Fabrizio Caldera 3 , Claudio Cecone 3 , Adrián Matencio 3 , Aliasgar F Shahiwala 1 , Francesco Trotta 3
|
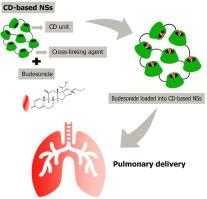
Budesonide (BUD) is a glucocorticosteroid used to treat chronic obstructive pulmonary disease. Despite this, it is a hydrophobic compound with low bioavailability. To address these hurdles, non-toxic and biocompatible βcyclodextrin-based nanosponges (βCD-NS) were attempted. BUD was loaded on five different βCD-NS at four different ratios. NS with 1,1′-carbonyldiimidazole (CDI) as a crosslinking agent, presented a higher encapsulation efficiency ( ̴ 80%) of BUD at 1:3 BUD: βCD-NS ratio (BUD-βCD-NS). The optimized formulations were characterized by Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), water absorption capacity (WAC), scanning electron microscopy (SEM), X-ray powder diffraction studies (XRD), particle size, zeta potential, encapsulation efficiency, in vitro and in vivo release studies, acute toxicity study, solid-state characterization, and aerosol performance. In vitro-in vivo correlation and cytotoxicity of the formulations on alveolar cells in vitro were further determined. In vitro and in vivo studies showed almost complete drug release and drug absorption from the lungs in the initial 2 h for pure BUD, which were sustained up to 12 h from BUD loaded into nanosponges (BUD-βCD-NS). Acute toxicity studies and in vitro cytotoxicity studies on alveolar cells proved the safety of BUD-βCD-NS. Several parameters, including particle size, median mass aerodynamic diameter, % fine particle fraction, and % emitted dose, were evaluated for aerosol performance, suggesting the capability of BUD-βCD-NS to formulate as a dry powder inhaler (DPI) with a suitable diluent. To sum up, this research will offer new insights into the future advancement of βCD-NS as drug delivery systems for providing controlled release of therapeutic agents against pulmonary disease.
中文翻译:

布地奈德肺部给药β环糊精纳米海绵的制备及评价
布地奈德(BUD)是一种糖皮质激素,用于治疗慢性阻塞性肺病。尽管如此,它是一种生物利用度较低的疏水性化合物。为了解决这些障碍,尝试了无毒且生物相容的基于β环糊精的纳米海绵(βCD-NS)。BUD 以四种不同的比例加载到五种不同的 βCD-NS 上。以 1,1′-羰基二咪唑 (CDI) 作为交联剂的 NS,在 BUD: βCD-NS 比例 (BUD-βCD-NS) 为 1:3 时表现出更高的 BUD 包封率 (̴ 80%)。通过傅里叶变换红外光谱 (FTIR)、热重分析 (TGA)、吸水能力 (WAC)、扫描电子显微镜 (SEM)、X 射线粉末衍射研究 (XRD)、粒径、zeta 电位对优化的配方进行表征、封装效率、体外和体内释放研究、急性毒性研究、固态表征和气溶胶性能。进一步确定了制剂在体外对肺泡细胞的体外-体内相关性和细胞毒性。体外和体内研究表明,纯 BUD 在最初 2 小时内几乎完全从肺部释放药物和吸收药物,而将 BUD 装入纳米海绵 (BUD-βCD-NS) 后,这种情况可持续长达 12 小时。肺泡细胞的急性毒性研究和体外细胞毒性研究证明了BUD-βCD-NS的安全性。对几个参数(包括粒径、中值质量空气动力学直径、细颗粒分数%和发射剂量%)进行了气雾剂性能评估,表明 BUD-βCD-NS 能够配制为具有合适配方的干粉吸入器 (DPI)。冲淡。总而言之,这项研究将为 βCD-NS 作为药物递送系统的未来发展提供新的见解,以提供针对肺部疾病的治疗药物的受控释放。































 京公网安备 11010802027423号
京公网安备 11010802027423号