Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2023-10-16 , DOI: 10.1016/j.jechem.2023.10.004
Min Sun , Luxiao Zhang , Fuli Tian , Jiaxin Li , Yanqiu Lei , Heng Zhang , Lifeng Han , Zhihua Guo , Yonghui Gao , Fenrong Liu , Yan Wang , Luhui Wang , Shanghong Zeng
|
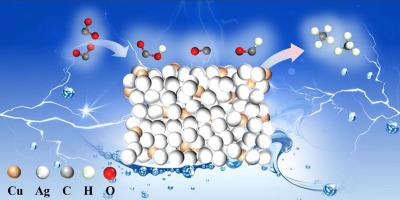
Silver-copper electrocatalysts have demonstrated effectively catalytic performance in electroreduction CO2 toward CH4, yet a revealing insight into the reaction pathway and mechanism has remained elusive. Herein, we construct chemically bonded Ag-Cu2O boundaries, in which the complete reduction of Cu2O to Cu has been strongly impeded owing to the presence of surface Ag shell. The interfacial confinement effect helps to maintain Cu+ sites at the Ag-Cu2O boundaries. Using in situ/operando spectroscopy and theoretical simulations, it is revealed that CO2 is enriched at the Ag-Cu2O boundaries due to the enhanced physisorption and chemisorption to CO2, activating CO2 to form the stable intermediate *CO. The boundaries between Ag shell and the Cu2O mediate local *CO coverage and promote *CHO intermediate formation, consequently facilitating CO2-to-CH4 conversion. This work not only reveals the structure-activity relationships but also offers insights into the reaction mechanism on Ag-Cu catalysts for efficient electrocatalytic CO2 reduction.
中文翻译:

通过原位/操作光谱和理论分析研究 Ag-Cu2O 电催化 CO2 制 CH4 的机理
银-铜电催化剂已在CO 2电还原成CH 4方面表现出有效的催化性能,但对反应途径和机制的揭示仍然难以捉摸。在此,我们构建了化学键合的Ag-Cu 2 O边界,其中由于表面Ag壳的存在, Cu 2 O完全还原为Cu受到强烈阻碍。界面限制效应有助于保持Ag-Cu 2 O边界处的Cu +位点。利用原位/操作光谱和理论模拟,揭示了由于对CO 2 的物理吸附和化学吸附增强,CO 2在Ag-Cu 2 O边界富集,活化CO 2形成稳定的中间体*CO。Ag壳和Cu 2 O之间的边界介导局部*CO覆盖并促进*CHO中间体形成,从而促进CO 2至CH 4转化。这项工作不仅揭示了结构-活性关系,还为Ag-Cu催化剂上有效电催化CO 2还原的反应机制提供了见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号