当前位置:
X-MOL 学术
›
Biomed. Pharmacother.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of murine colorectal cancer metastasis by targeting M2-TAM through STAT3/NF-kB/AKT signaling using macrophage 1-derived extracellular vesicles loaded with oxaliplatin, retinoic acid, and Libidibia ferrea
Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2023-10-11 , DOI: 10.1016/j.biopha.2023.115663 Thaís Gomes de Carvalho 1 , Pablo Lara 2 , Carla Jorquera-Cordero 3 , Cícero Flávio Soares Aragão 4 , Artur de Santana Oliveira 4 , Vinicius Barreto Garcia 5 , Shirley Vitória de Paiva Souza 6 , Timo Schomann 2 , Luiz Alberto Lira Soares 7 , Paulo Marcos da Matta Guedes 8 , Raimundo Fernandes de Araújo Júnior 1
Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2023-10-11 , DOI: 10.1016/j.biopha.2023.115663 Thaís Gomes de Carvalho 1 , Pablo Lara 2 , Carla Jorquera-Cordero 3 , Cícero Flávio Soares Aragão 4 , Artur de Santana Oliveira 4 , Vinicius Barreto Garcia 5 , Shirley Vitória de Paiva Souza 6 , Timo Schomann 2 , Luiz Alberto Lira Soares 7 , Paulo Marcos da Matta Guedes 8 , Raimundo Fernandes de Araújo Júnior 1
Affiliation
|
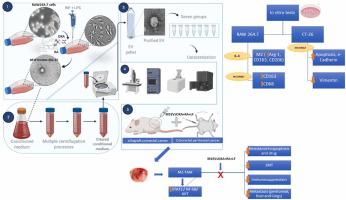
Colorectal cancer is still unmanageable despite advances in target therapy. However, extracellular vesicles (EVs) have shown potential in nanomedicine as drug delivery systems, especially for modulating the immune cells in the tumor microenvironment (TME). In this study, M1 Macrophage EVs (M1EVs) were used as nanocarriers of oxaliplatin (M1EV1) associated with retinoic acid (M1EV2) and (M1EV3), alone or in combination (M1EV4) to evaluate their antiproliferative and immunomodulatory potential on CT-26 and MC-38 colorectal cancer cell lines and prevent metastasis in mice of allograft and peritoneal colorectal cancer models. Tumors were evaluated by qRT-PCR and immunohistochemistry. The cell death profile and epithelial-mesenchymal transition process (EMT) were analyzed in vitro in colorectal cancer cell lines. Polarization of murine macrophages (RAW264.7 cells) was also carried out. M1EV2 and M1EV3 used alone or particularly M1EV4 downregulated the tumor progression by TME immunomodulation, leading to a decrease in primary tumor size and metastasis in the peritoneum, liver, and lungs. STAT3, NF-kB, and AKT were the major genes downregulated by of M1EV systems. Tumor-associated macrophages (TAMs) shifted from an M2 phenotype (CD163) to an M1 phenotype (CD68) reducing levels of IL-10, TGF-β and CCL22. Furthermore, malignant cells showed overexpression of FADD, APAF-1, caspase-3, and E-cadherin, and decreased expression of MDR1, survivin, vimentin, and PD-L1 after treatment with systems of M1EVs. The study shows that EVs from M1 antitumor macrophages can transport drugs and enhance their immunomodulatory and antitumor activity by modulating pathways associated with cell proliferation, migration, survival, and drug resistance.
中文翻译:

使用负载奥沙利铂、视黄酸和利比迪比亚铁的巨噬细胞 1 衍生的细胞外囊泡,通过 STAT3/NF-kB/AKT 信号传导靶向 M2-TAM,抑制小鼠结直肠癌转移
尽管靶向治疗取得了进展,结直肠癌仍然难以控制。然而,细胞外囊泡(EV)在纳米医学中显示出作为药物递送系统的潜力,特别是在调节肿瘤微环境(TME)中的免疫细胞方面。在这项研究中,M1巨噬细胞EV(M1EV)被用作奥沙利铂(M1EV1)与视黄酸(M1EV2)和(M1EV3)单独或组合(M1EV4)的纳米载体,以评估其对CT-26和CT-26的抗增殖和免疫调节潜力。 MC-38 结直肠癌细胞系并预防同种异体移植小鼠和腹膜结直肠癌模型的转移。通过 qRT-PCR 和免疫组织化学评估肿瘤。在体外分析结直肠癌细胞系的细胞死亡概况和上皮间质转化过程(EMT)。还进行了小鼠巨噬细胞(RAW264.7 细胞)的极化。单独使用 M1EV2 和 M1EV3 或特别是 M1EV4 通过 TME 免疫调节下调肿瘤进展,导致原发肿瘤尺寸减小以及腹膜、肝脏和肺部的转移。 STAT3、NF-kB和AKT是M1EV系统下调的主要基因。肿瘤相关巨噬细胞 (TAM) 从 M2 表型 (CD163) 转变为 M1 表型 (CD68),降低了 IL-10、TGF-β 和 CCL22 的水平。此外,用 M1EV 系统处理后,恶性细胞表现出 FADD、APAF-1、caspase-3 和 E-cadherin 的过度表达,并且 MDR1、survivin、vimentin 和 PD-L1 的表达降低。研究表明,来自 M1 抗肿瘤巨噬细胞的 EV 可以通过调节与细胞增殖、迁移、存活和耐药性相关的途径来运输药物并增强其免疫调节和抗肿瘤活性。
更新日期:2023-10-11
中文翻译:

使用负载奥沙利铂、视黄酸和利比迪比亚铁的巨噬细胞 1 衍生的细胞外囊泡,通过 STAT3/NF-kB/AKT 信号传导靶向 M2-TAM,抑制小鼠结直肠癌转移
尽管靶向治疗取得了进展,结直肠癌仍然难以控制。然而,细胞外囊泡(EV)在纳米医学中显示出作为药物递送系统的潜力,特别是在调节肿瘤微环境(TME)中的免疫细胞方面。在这项研究中,M1巨噬细胞EV(M1EV)被用作奥沙利铂(M1EV1)与视黄酸(M1EV2)和(M1EV3)单独或组合(M1EV4)的纳米载体,以评估其对CT-26和CT-26的抗增殖和免疫调节潜力。 MC-38 结直肠癌细胞系并预防同种异体移植小鼠和腹膜结直肠癌模型的转移。通过 qRT-PCR 和免疫组织化学评估肿瘤。在体外分析结直肠癌细胞系的细胞死亡概况和上皮间质转化过程(EMT)。还进行了小鼠巨噬细胞(RAW264.7 细胞)的极化。单独使用 M1EV2 和 M1EV3 或特别是 M1EV4 通过 TME 免疫调节下调肿瘤进展,导致原发肿瘤尺寸减小以及腹膜、肝脏和肺部的转移。 STAT3、NF-kB和AKT是M1EV系统下调的主要基因。肿瘤相关巨噬细胞 (TAM) 从 M2 表型 (CD163) 转变为 M1 表型 (CD68),降低了 IL-10、TGF-β 和 CCL22 的水平。此外,用 M1EV 系统处理后,恶性细胞表现出 FADD、APAF-1、caspase-3 和 E-cadherin 的过度表达,并且 MDR1、survivin、vimentin 和 PD-L1 的表达降低。研究表明,来自 M1 抗肿瘤巨噬细胞的 EV 可以通过调节与细胞增殖、迁移、存活和耐药性相关的途径来运输药物并增强其免疫调节和抗肿瘤活性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号