Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2023-10-13 , DOI: 10.1016/j.apcata.2023.119459 Lu Ji , Fang Li , Conghui Che , Wei Xue , Qiusheng Yang , Xiaoshu Ding , Dongsheng Zhang , Xinqiang Zhao , Yanji Wang
|
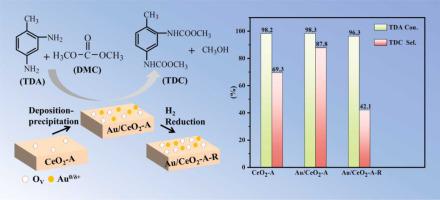
In the reaction of dimethyl carbonate (DMC) and 2,4-diaminotoluene (TDA), Au/CeO2 exhibits higher selectivity than CeO2 for dimethyl toluene-2,4-dicarbamate (TDC). However, its catalytic activity drops after H2 reduction (denoted as Au/CeO2-R). The characterization results illustrate that Au exists as a single atom with the primary species as Auδ+ in Au/CeO2, forming CexAu1−xO2 structure similar to solid solution. Whereas for Au/CeO2-R catalyst, Au mainly exists as Au0, forming the Au-O-Ce structure on CeO2 surface. Significantly, the strong interaction between Au and CeO2 increases Ce3+ and oxygen vacancies content, as well as modulates their acidic property. However, the catalytic activity of Au/CeO2-R is found to be lower than Au/CeO2 as the species formed by DMC dissociation on its surface are not easily desorbed. Other transition metals supported on CeO2 are also prepared, wherein Pt/CeO2 and Pd/CeO2 exhibit good TDC selectivity. In the end, the electronegativity and catalytic activity of metals are correlated.
中文翻译:

单原子Au修饰CeO2催化剂的结构及其在2,4-二氨基甲苯甲氧基羰基化反应中的催化性能
在碳酸二甲酯(DMC)和2,4-二氨基甲苯(TDA)的反应中,Au/CeO 2对甲苯-2,4-二氨基甲酸二甲酯(TDC)表现出比CeO 2更高的选择性。然而,H 2还原后其催化活性下降(表示为Au/CeO 2 -R)。表征结果表明,Au在Au/CeO 2中以单原子形式存在,主要形态为Au δ+,形成类似固溶体的C x Au 1−x O 2结构。而对于Au/CeO 2 -R催化剂,Au主要以Au 0 的形式存在,在CeO 2表面形成Au-O-Ce结构。值得注意的是,Au 和 CeO 2之间的强相互作用增加了 Ce 3+和氧空位含量,并调节了它们的酸性。然而,发现Au/CeO 2 -R的催化活性低于Au/CeO 2,因为DMC在其表面解离形成的物质不易解吸。还制备了负载在CeO 2上的其他过渡金属,其中Pt/CeO 2和Pd/CeO 2表现出良好的TDC选择性。最后,金属的电负性和催化活性是相关的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号