当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating the oxidation mechanism of facet-dependent pyrite: implications for the environment and sulfur evolution
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2023-10-16 , DOI: 10.1039/d3em00221g Chenrui Liu 1 , Yun Liu 1 , Shuai Zeng 1 , Dejian Li 1
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2023-10-16 , DOI: 10.1039/d3em00221g Chenrui Liu 1 , Yun Liu 1 , Shuai Zeng 1 , Dejian Li 1
Affiliation
|
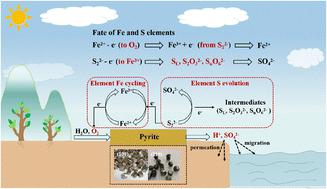
The oxidation of pyrite (FeS2) not only adversely affects the environment, but also plays a critical role in the geochemical evolution of Fe and S elements. However, the oxidation rate of FeS2 is often controlled by its exposed crystal facets. Herein, the oxidation behaviors and mechanisms of naturally existing FeS2(100) and FeS2(210) crystals are investigated. The adsorption models of O2 on FeS2(100) and FeS2(210) facets are established, additionally, their corresponding surface energies, O2 adsorption sites and energies are also obtained using Density Functional Theory (DFT) calculations. These results suggest that the FeS2(210) facet more readily reacts with O2 because it has more unsaturated coordination of Fe atoms compared with the FeS2(100) facet. Moreover, electrochemical results such as EIS, Tafel and CV curves further prove that FeS2(210) possesses a higher oxidation rate than that of FeS2(100). The results of chemical oxidation experiments and XPS analyses show that FeS2(210) can produce more total Fe, SO42− and H+ than FeS2(100). Furthermore, various intermediate S species such as SO32−, S2O32−, S3O62−, S4O62− and S5O62− are also detected. This work can provide a basis for understanding the oxidation mechanism of facet-dependent FeS2 and the geochemical evolution of Fe and S elements.
中文翻译:

研究晶面相关黄铁矿的氧化机制:对环境和硫演化的影响
黄铁矿(FeS 2 )的氧化不仅对环境产生不利影响,而且在Fe和S元素的地球化学演化中起着至关重要的作用。然而,FeS 2的氧化速率通常由其暴露的晶面控制。在此,研究了天然存在的FeS 2 (100)和FeS 2 (210)晶体的氧化行为和机制。建立了O 2在FeS 2 (100)和FeS 2 (210)晶面上的吸附模型,并利用密度泛函理论(DFT)计算得到了它们相应的表面能、O 2吸附位点和能量。这些结果表明,FeS 2 (210) 面更容易与O 2反应,因为与FeS 2 (100) 面相比,FeS 2 (210) 面具有更多的Fe原子不饱和配位。此外,EIS、Tafel和CV曲线等电化学结果进一步证明FeS 2 (210)比FeS 2 (100)具有更高的氧化速率。化学氧化实验和XPS分析结果表明,FeS 2 (210)比FeS 2 (100)能产生更多的总Fe、SO 4 2−和H + 。 此外,还检测到各种中间S物种,例如SO 3 2− 、S 2 O 3 2− 、S 3 O 6 2− 、S 4 O 6 2−和S 5 O 6 2− 。该工作可为理解晶面依赖性FeS 2的氧化机制以及Fe和S元素的地球化学演化提供基础。
更新日期:2023-10-16
中文翻译:

研究晶面相关黄铁矿的氧化机制:对环境和硫演化的影响
黄铁矿(FeS 2 )的氧化不仅对环境产生不利影响,而且在Fe和S元素的地球化学演化中起着至关重要的作用。然而,FeS 2的氧化速率通常由其暴露的晶面控制。在此,研究了天然存在的FeS 2 (100)和FeS 2 (210)晶体的氧化行为和机制。建立了O 2在FeS 2 (100)和FeS 2 (210)晶面上的吸附模型,并利用密度泛函理论(DFT)计算得到了它们相应的表面能、O 2吸附位点和能量。这些结果表明,FeS 2 (210) 面更容易与O 2反应,因为与FeS 2 (100) 面相比,FeS 2 (210) 面具有更多的Fe原子不饱和配位。此外,EIS、Tafel和CV曲线等电化学结果进一步证明FeS 2 (210)比FeS 2 (100)具有更高的氧化速率。化学氧化实验和XPS分析结果表明,FeS 2 (210)比FeS 2 (100)能产生更多的总Fe、SO 4 2−和H + 。 此外,还检测到各种中间S物种,例如SO 3 2− 、S 2 O 3 2− 、S 3 O 6 2− 、S 4 O 6 2−和S 5 O 6 2− 。该工作可为理解晶面依赖性FeS 2的氧化机制以及Fe和S元素的地球化学演化提供基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号