Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Thiophenyl-pyrazolyl-thiazole Hybrids as DHFR Inhibitors: Design, Synthesis, Antimicrobial Evaluation, Molecular Modeling, and Biodistribution Studies
ACS Omega ( IF 3.7 ) Pub Date : 2023-10-13 , DOI: 10.1021/acsomega.3c04736 Dina H Dawood 1 , Manal M Sayed 2 , Sally T K Tohamy 3 , Eman S Nossier 4, 5
ACS Omega ( IF 3.7 ) Pub Date : 2023-10-13 , DOI: 10.1021/acsomega.3c04736 Dina H Dawood 1 , Manal M Sayed 2 , Sally T K Tohamy 3 , Eman S Nossier 4, 5
Affiliation
|
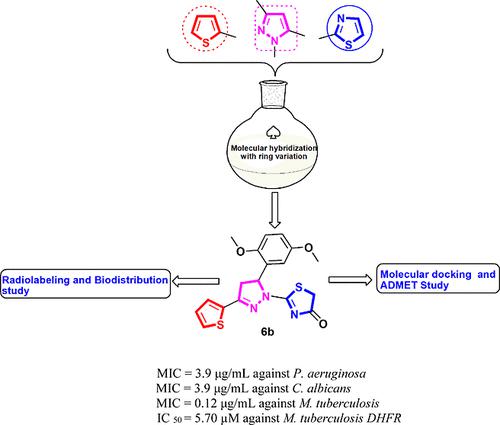
The antibiotic resistance problems constitute a considerable threat to human health worldwide; thus, the discovery of new antimicrobial candidates to conquer this issue is an imperative requirement. From this view, new thiophenyl-pyrazolyl-thiazole hybrids 3–10 were synthesized and screened for their antibacterial efficiency versus Gram – and Gram + bacterial strains compared to the reference drug amoxicillin. It was noticed that the new hybrids displayed significant antibacterial efficacy versus Gram – bacteria, especially against Pseudomonas aeruginosa. Also, all the screened candidates demonstrated a noticeable antifungal effect against Candida albicans (MICs = 3.9–125 μg/mL) relative to fluconazole (MIC = 250 μg/mL). Moreover, the new hybrids were investigated for their antituberculosis potency against Mycobacterium tuberculosis (RCMB 010126). Derivatives 4c, 6b, 8b, 9b, and 10b demonstrated prominent antituberculosis efficiency (MICs = 0.12–1.95 μg/mL) compared with the reference drug isoniazid (MIC = 0.12 μg/mL). The latter derivatives were further assessed for their inhibitory potency versus M. tuberculosis DHFR enzyme. The compounds 4c, 6b and 10b presented a remarkable suppression effect with IC50 values of 4.21, 5.70, and 10.59 μM, respectively, compared to that of trimethoprim (IC50 = 6.23 μM). Furthermore, biodistribution profile using radiolabeling way revealed a perceived uptake of 131I-compound 6b into infection induced models. The docking study for the new hybrids 4c, 6b, 8b, 9b and 10b was performed to illustrate the various binding modes with Mtb DHFR enzyme. In silico ADMET studies for the most potent inhibitors 4c, 6b and 10b were also accomplished to predict their pharmacokinetic and physicochemical features.
中文翻译:

作为 DHFR 抑制剂的新型噻吩基-吡唑基-噻唑杂化物:设计、合成、抗菌评价、分子建模和生物分布研究
抗生素耐药性问题对全世界人类健康构成相当大的威胁;因此,发现新的抗菌药物候选物来解决这一问题势在必行。从这个角度来看,合成了新的噻吩基-吡唑基-噻唑杂化物3-10,并筛选了与参考药物阿莫西林相比,其对革兰氏 - 和革兰氏 + 菌株的抗菌效率。人们注意到,新的杂交体对革兰氏菌,特别是对铜绿假单胞菌表现出显着的抗菌功效。此外,相对于氟康唑 (MIC = 250 μg/mL),所有筛选的候选药物均表现出对白色念珠菌(MIC = 3.9–125 μg/mL) 的显着抗真菌作用。此外,还研究了新杂交种对结核分枝杆菌(RCMB 010126)的抗结核功效。与参考药物异烟肼 (MIC = 0.12 μg/mL) 相比,衍生物4c、6b、8b、9b和10b表现出显着的抗结核功效 (MIC = 0.12–1.95 μg/mL)。进一步评估了后一种衍生物对结核分枝杆菌DHFR 酶的抑制效力。与甲氧苄啶(IC 50 = 6.23 μM)相比,化合物4c、6b和10b表现出显着的抑制效果,IC 50值分别为4.21、5.70和10.59 μM。此外,使用放射性标记方式的生物分布概况揭示了感染诱导模型中131 I-化合物6b的感知摄取。对新杂交体4c、6b、8b、9b和10b进行对接研究,以说明与Mtb DHFR 酶的各种结合模式。还对最有效的抑制剂4c、6b和10b进行了计算机 ADMET 研究,以预测其药代动力学和理化特征。
更新日期:2023-10-13
中文翻译:

作为 DHFR 抑制剂的新型噻吩基-吡唑基-噻唑杂化物:设计、合成、抗菌评价、分子建模和生物分布研究
抗生素耐药性问题对全世界人类健康构成相当大的威胁;因此,发现新的抗菌药物候选物来解决这一问题势在必行。从这个角度来看,合成了新的噻吩基-吡唑基-噻唑杂化物3-10,并筛选了与参考药物阿莫西林相比,其对革兰氏 - 和革兰氏 + 菌株的抗菌效率。人们注意到,新的杂交体对革兰氏菌,特别是对铜绿假单胞菌表现出显着的抗菌功效。此外,相对于氟康唑 (MIC = 250 μg/mL),所有筛选的候选药物均表现出对白色念珠菌(MIC = 3.9–125 μg/mL) 的显着抗真菌作用。此外,还研究了新杂交种对结核分枝杆菌(RCMB 010126)的抗结核功效。与参考药物异烟肼 (MIC = 0.12 μg/mL) 相比,衍生物4c、6b、8b、9b和10b表现出显着的抗结核功效 (MIC = 0.12–1.95 μg/mL)。进一步评估了后一种衍生物对结核分枝杆菌DHFR 酶的抑制效力。与甲氧苄啶(IC 50 = 6.23 μM)相比,化合物4c、6b和10b表现出显着的抑制效果,IC 50值分别为4.21、5.70和10.59 μM。此外,使用放射性标记方式的生物分布概况揭示了感染诱导模型中131 I-化合物6b的感知摄取。对新杂交体4c、6b、8b、9b和10b进行对接研究,以说明与Mtb DHFR 酶的各种结合模式。还对最有效的抑制剂4c、6b和10b进行了计算机 ADMET 研究,以预测其药代动力学和理化特征。







































 京公网安备 11010802027423号
京公网安备 11010802027423号