当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Roles of bridge carbon and heteroatom during the dehydrogenation reaction of dicyclohexylmethane derivatives on Pd and Pt catalysts for liquid organic hydrogen carrier
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-10-13 , DOI: 10.1016/j.apcatb.2023.123394 Hyunwoo Yook , Kwanyong Jeong , Jinwoo Hwang , Ji Hoon Park , Jeong Woo Han
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-10-13 , DOI: 10.1016/j.apcatb.2023.123394 Hyunwoo Yook , Kwanyong Jeong , Jinwoo Hwang , Ji Hoon Park , Jeong Woo Han
|
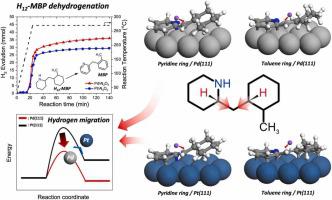
Liquid organic hydrogen carrier (LOHC) has been developed to overcome the low efficiency of conventional hydrogen storage methods. Although 2-[-methylbenzyl]pyridine (MBP), one of dicyclohexylmethane derivatives, has been reported as a high-performance LOHC material, its dehydrogenation mechanism has yet to be fully understood. Here, we combine both experiments and theoretical calculations to comparatively elucidate the dehydrogenation mechanism of H-MBP on Pd and Pt catalysts. The experimental results for hydrogen release from H-MBP and H-MBP show that Pd/AlO has a faster initial hydrogen release rate than Pt/AlO. Density functional theory calculations reveal that the N-heteroatom in the pyridine ring has a significant effect on the adsorption of H-MBP. In addition, the hydrogen migration in the pyridine ring serves as the rate-determining step, where the activation free energy of Pd(111) is lower than that of Pt(111). Our results provide insight into the dehydrogenation of heterocyclic compounds with a bridge structure on metal catalysts.
中文翻译:

液态有机氢载体 Pd 和 Pt 催化剂上二环己基甲烷衍生物脱氢反应中桥碳和杂原子的作用
液态有机氢载体(LOHC)的开发是为了克服传统储氢方法效率低的问题。尽管二环己基甲烷衍生物之一2-[-甲基苄基]吡啶(MBP)已被报道为一种高性能LOHC材料,但其脱氢机制尚未完全了解。在这里,我们结合实验和理论计算来比较阐明H-MBP在Pd和Pt催化剂上的脱氢机理。 H-MBP和H-MBP的放氢实验结果表明,Pd/Al2O比Pt/Al2O具有更快的初始放氢速率。密度泛函理论计算表明吡啶环中的N-杂原子对H-MBP的吸附有显着影响。此外,吡啶环中的氢迁移是速率决定步骤,其中Pd(111)的活化自由能低于Pt(111)的活化自由能。我们的结果提供了对金属催化剂上具有桥结构的杂环化合物脱氢的深入了解。
更新日期:2023-10-13
中文翻译:

液态有机氢载体 Pd 和 Pt 催化剂上二环己基甲烷衍生物脱氢反应中桥碳和杂原子的作用
液态有机氢载体(LOHC)的开发是为了克服传统储氢方法效率低的问题。尽管二环己基甲烷衍生物之一2-[-甲基苄基]吡啶(MBP)已被报道为一种高性能LOHC材料,但其脱氢机制尚未完全了解。在这里,我们结合实验和理论计算来比较阐明H-MBP在Pd和Pt催化剂上的脱氢机理。 H-MBP和H-MBP的放氢实验结果表明,Pd/Al2O比Pt/Al2O具有更快的初始放氢速率。密度泛函理论计算表明吡啶环中的N-杂原子对H-MBP的吸附有显着影响。此外,吡啶环中的氢迁移是速率决定步骤,其中Pd(111)的活化自由能低于Pt(111)的活化自由能。我们的结果提供了对金属催化剂上具有桥结构的杂环化合物脱氢的深入了解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号