Synthesis ( IF 2.2 ) Pub Date : 2023-10-13 , DOI: 10.1055/a-2164-2075 Mario Ordónez 1, 2 , Rubén Oswaldo Argüello-Velasco 3 , Ivan Romero-Estudillo 4 , Victoria Labastida-Galván 5 , Teodoro Miranda-Blancas 5
|
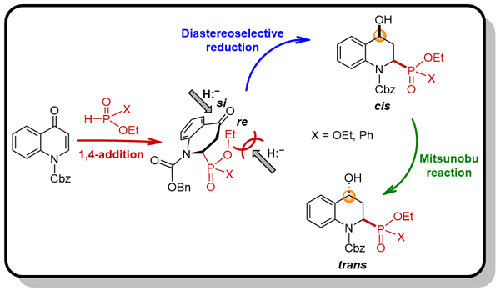
We report a practical method for the first stereoselective synthesis of diethyl cis- and trans-(4-hydroxy-1,2,3,4-tetrahydroquinolin-2-yl)phosphonates, as well as ethyl cis- and trans-(4-hydroxy-1,2,3,4-tetrahydroquinolin-2-yl)phenylphosphinates. The main feature of this method is the regioselective 1,4-phosponylation to N-Cbz quinolin-4(1H)-one using diethyl phosphite or ethyl phenylphosphinate followed by a highly diastereoselective reduction to give the cis stereoisomers as favored products, which were converted into trans stereoisomers through the Mitsunobu reaction. Cleavage of the N-Cbz bond under hydrogenolysis gave the target heterocyclic α-aminophosphonates and α-aminophosphinates.
中文翻译:

首次以喹啉-4(1H)-酮为原料立体选择性合成顺反式(4-羟基-1,2,3,4-四氢喹啉-2-基)膦酸二乙酯和苯基次膦酸乙酯
我们报告了一种实用方法,用于首次立体选择性合成顺式和反式-(4-羟基-1,2,3,4-四氢喹啉-2-基)膦酸二乙酯,以及顺式和反式-(4-羟基-1,2,3,4-四氢喹啉-2-基)苯基次膦酸酯。该方法的主要特点是使用亚磷酸二乙酯或苯基次膦酸乙酯进行区域选择性1,4-膦酰化为N -Cbz quinolin-4(1 H )-one,然后进行高度非对映选择性还原,得到顺式立体异构体作为首选产物,通过光延反应转化为反式立体异构体。N -Cbz 键在氢解作用下断裂,得到目标杂环 α-氨基膦酸酯和 α-氨基次膦酸酯。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号